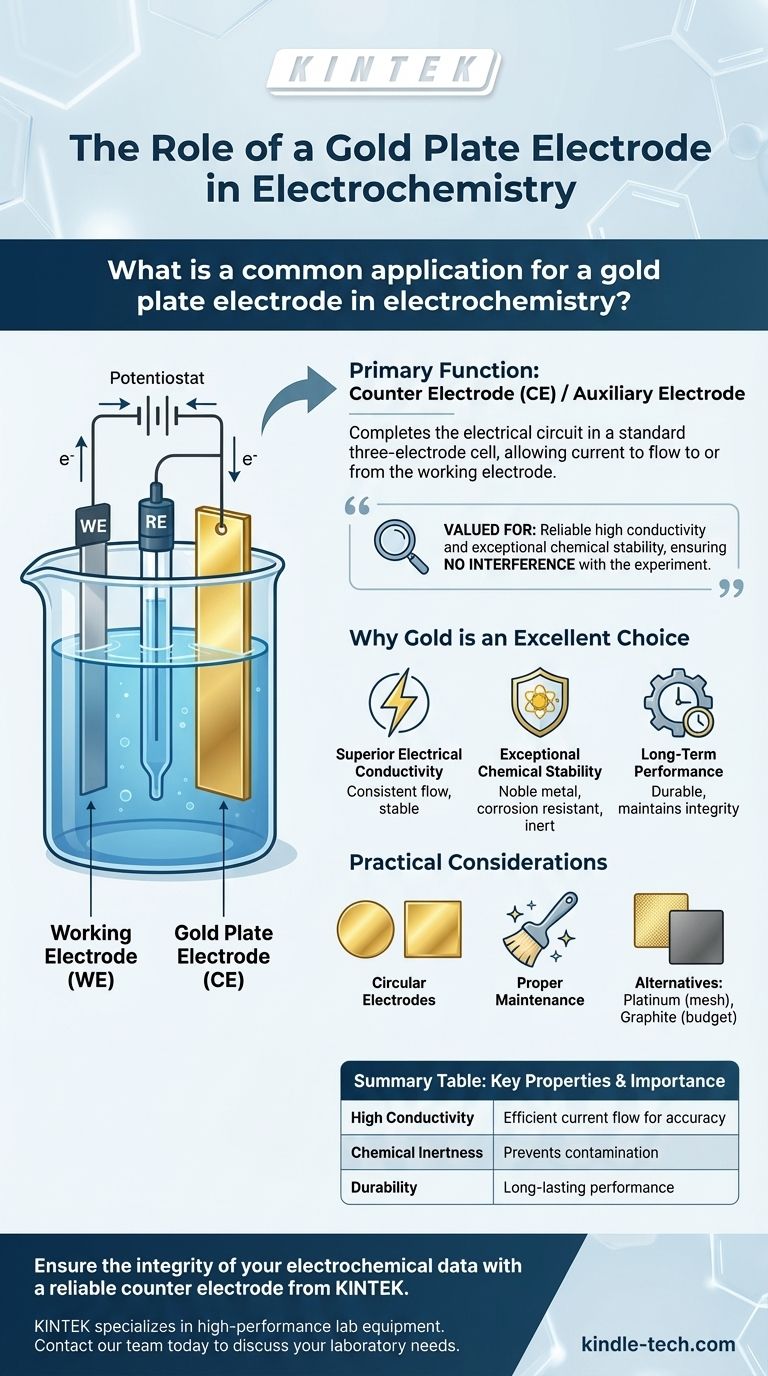
In electrochemistry, a gold plate electrode is most commonly used as a Counter Electrode (CE), also known as an auxiliary electrode. Its primary function is to complete the electrical circuit in a standard three-electrode cell, allowing current to flow to or from the working electrode where the reaction of interest is taking place.
A gold plate electrode is valued not for participating in the main reaction, but for its exact opposite quality: its ability to reliably pass current with high conductivity and exceptional chemical stability, ensuring it does not interfere with the experiment being measured.

The Role of the Counter Electrode
In modern electrochemistry, most experiments rely on a three-electrode setup to get accurate measurements. Understanding the role of each component is key to understanding why gold is a preferred material.
The Three-Electrode System
A three-electrode cell consists of a Working Electrode (WE), where the primary chemical reaction you are studying occurs. It also includes a Reference Electrode (RE), which provides a stable, constant potential that all other potentials are measured against.
The third component is the Counter Electrode (CE).
The Function of the Counter Electrode
The counter electrode's job is to act as a source or sink for electrons, effectively balancing the charge. It completes the electrical circuit between the power source (potentiostat) and the solution, ensuring that the current flowing through the working electrode is accurately controlled and measured.
Why Its Material Matters
The material of the counter electrode must be chosen carefully. It needs to be highly conductive to pass current efficiently and, most importantly, it must be chemically inert so it doesn't corrode, dissolve, or create byproducts that could interfere with the sensitive reaction happening at the working electrode.
Why Gold is an Excellent Choice for this Role
Gold possesses a unique combination of physical and chemical properties that make it an ideal material for a high-performance counter electrode.
Superior Electrical Conductivity
A gold plate electrode has highly stable electrical conductivity. This property shows minimal variation under different temperatures or environmental conditions, ensuring a consistent and reliable flow of current throughout the experiment.
Exceptional Chemical Stability
Gold is a noble metal, meaning it has excellent corrosion resistance. It is not easily oxidized or corroded in most electrochemical environments. This chemical inertness is critical, as it guarantees the electrode itself will not contaminate the experiment.
Long-Term Performance
This combination of conductivity and stability ensures that the electrode maintains its structural integrity and performance over many uses, making it a reliable and long-lasting tool in the laboratory.
Practical Considerations and Trade-offs
While gold is an excellent material, its use comes with practical considerations regarding its form, care, and cost.
Physical Forms and Customization
Gold plate electrodes are available in various shapes (circular, square, rectangular) and thicknesses, typically from 0.1 mm to 0.5 mm. Their surfaces can be polished or modified for specific applications, offering versatility for different experimental setups.
The Importance of Proper Maintenance
To ensure longevity, the electrode requires proper care. This includes regular cleaning and inspection. For storage, it must be dried thoroughly and kept in a dedicated, protected container away from air.
Common Alternatives
Platinum is another noble metal frequently used for counter electrodes, often in a mesh form. Like gold, it offers excellent conductivity and stability. The choice between gold and platinum often depends on the specific chemical environment of the experiment, as one may be slightly more inert than the other in a given solution.
The Cost Factor
The primary trade-off for using gold or platinum is cost. For less sensitive or general-purpose experiments where absolute inertness is not critical, more affordable materials like graphite can serve as a suitable counter electrode.
Making the Right Choice for Your Experiment
Selecting the appropriate counter electrode is a foundational step in designing a reliable electrochemical experiment.
- If your primary focus is maximum data integrity and reliability: A gold or platinum plate electrode is the professional standard for ensuring your results are not compromised by electrode interference.
- If your experiment involves a specific chemical environment: Carefully choose between gold and platinum based on which material has the lowest reactivity with your specific electrolyte and analytes.
- If you are conducting educational or less sensitive experiments: A more budget-friendly material like a graphite rod may be a perfectly suitable and cost-effective alternative.
Ultimately, choosing the right counter electrode material is fundamental to ensuring your experimental results are both accurate and reproducible.
Summary Table:
| Property | Why It Matters for a Counter Electrode |
|---|---|
| High Conductivity | Ensures efficient and stable current flow for accurate measurements. |
| Chemical Inertness | Prevents corrosion and contamination of the experiment. |
| Durability | Provides long-lasting performance and reliability. |
Ensure the integrity of your electrochemical data with a reliable counter electrode from KINTEK.
Choosing the right lab equipment is fundamental to achieving accurate and reproducible results. KINTEK specializes in high-performance lab equipment and consumables, including gold plate electrodes, designed to meet the rigorous demands of your research.
Our electrodes offer the superior conductivity and chemical stability you need to prevent experimental interference. Let our experts help you select the perfect components for your specific application.
Contact our team today to discuss your laboratory needs and enhance your experimental setup.
Visual Guide
Related Products
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
People Also Ask
- What are the performance characteristics of a gold plate electrode? Unmatched Stability for Reliable Data
- How should a gold disc electrode be maintained for long-term use? A Guide to Consistent Performance
- What are the necessary pretreatment steps before using a gold disc electrode? A Guide to Reliable Electrochemical Data
- How should a gold disc electrode be handled during an experiment? Ensure Accurate Electrochemical Measurements
- What is the material and purity of a gold disc electrode? Ensuring Precision in Electrochemical Analysis



















