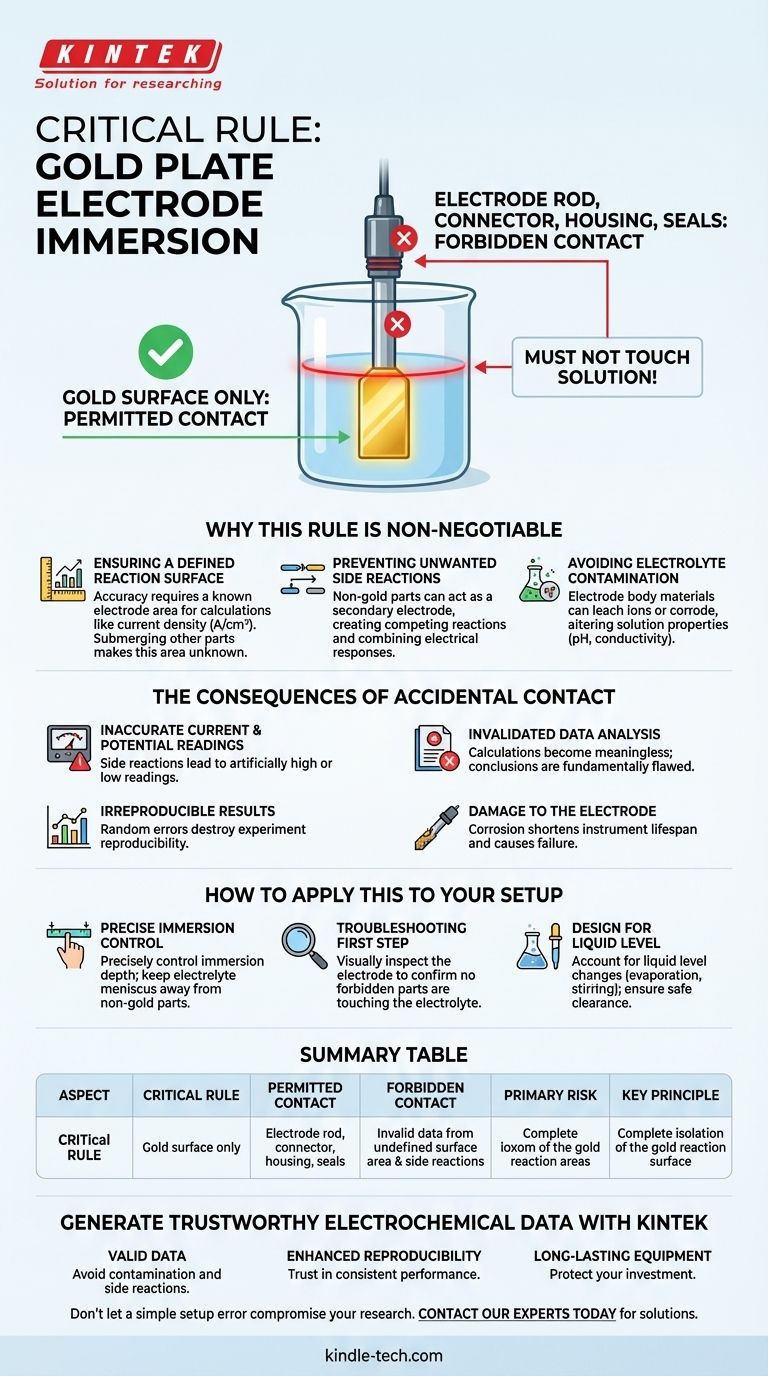
The single most critical rule for using a gold plate electrode is that only the gold surface itself must ever come into contact with the electrolyte solution. All other components, including the electrode rod, connector, and any encapsulated or sealed sections, are strictly forbidden from touching the solution. This is not a suggestion; it is a requirement for valid experimental results.
The core principle is one of isolation. Your experiment is designed to measure the electrochemical reaction occurring exclusively on the known surface of the gold. Any contact from other parts introduces unknown variables, side reactions, and contaminants that will fundamentally invalidate your data.

Why This Rule is Non-Negotiable
An electrochemical setup is a highly controlled system. The objective is to study the interaction between a specific electrode material (gold) and the electrolyte. Violating the immersion rule breaks this controlled system in several critical ways.
Ensuring a Defined Reaction Surface
The entire basis of quantitative electrochemistry relies on a known, well-defined electrode surface area. All calculations, such as current density (current per unit area), depend on this value being accurate.
When non-gold parts are submerged, the active electrochemical surface area becomes an unknown quantity. The resulting data cannot be normalized or compared reliably against other experiments.
Preventing Unwanted Side Reactions
The rod and housing of the electrode are often made of different materials that are not intended to be electrochemically inert in your specific electrolyte.
If these parts touch the solution, they can act as a second, parallel electrode. This creates competing electrochemical reactions that interfere with the primary reaction you are trying to measure on the gold surface. Your instrument will read the combined electrical response, not the isolated behavior of the gold.
Avoiding Electrolyte Contamination
The materials used for the electrode body and seals are not designed to withstand prolonged exposure to a potentially corrosive electrolyte.
These materials can leach impurities or corrode, releasing unwanted ions into your solution. This contamination can alter the electrolyte's pH, conductivity, or chemical composition, changing the very conditions you aim to study.
The Consequences of Accidental Contact
Failing to adhere to the immersion rule doesn't just introduce minor errors; it can render your entire experiment worthless. Understanding the specific consequences highlights the importance of a proper setup.
Inaccurate Current and Potential Readings
Side reactions on the submerged rod or housing will draw or contribute their own current. This leads to artificially high or low readings that do not accurately reflect the processes occurring on the gold surface.
Invalidated Data Analysis
Since key calculations like current density (A/cm²) become meaningless when the surface area ('cm²') is incorrect, any subsequent analysis or conclusions drawn from this data will be fundamentally flawed.
Irreproducible Results
If the immersion depth of the non-gold parts varies even slightly between experiments, you will introduce a random error that destroys the reproducibility of your work. This makes it impossible to draw reliable conclusions or compare different tests.
Damage to the Electrode
Submerging parts not designed for chemical exposure can lead to their degradation. This shortens the lifespan of a costly precision instrument and can cause it to fail mid-experiment.
How to Apply This to Your Setup
Proper electrode immersion is a matter of procedural discipline. Careful setup is the foundation of reliable electrochemical data.
- If your primary focus is accuracy and reproducibility: You must precisely control the immersion depth so that only the gold surface is submerged and the electrolyte's meniscus is well below any other component.
- If you are troubleshooting unexpected results: Your first diagnostic step should always be to visually inspect the electrode in the cell and confirm that no part of the housing, rod, or seal is touching the electrolyte.
- When designing any experiment: Always account for the liquid level. Ensure your cell and electrolyte volume allow the gold plate to be fully submerged while keeping all other parts safely above the surface, even with potential evaporation or stirring.
Mastering this fundamental rule is the first step toward generating trustworthy and publishable electrochemical data.
Summary Table:
| Aspect | Critical Rule |
|---|---|
| Permitted Contact | Gold surface only |
| Forbidden Contact | Electrode rod, connector, housing, seals |
| Primary Risk | Invalid data from undefined surface area & side reactions |
| Key Principle | Complete isolation of the gold reaction surface |
Generate Trustworthy Electrochemical Data with KINTEK
Ensuring precise electrode setup is fundamental to your lab's success. KINTEL specializes in high-quality lab equipment and consumables, including reliable electrochemical cells and electrodes designed for accurate, reproducible results.
Let us help you achieve:
- Valid Data: Avoid the pitfalls of contamination and side reactions with equipment built for precision.
- Enhanced Reproducibility: Trust in consistent performance experiment after experiment.
- Long-Lasting Equipment: Protect your investment with durable, corrosion-resistant components.
Don't let a simple setup error compromise your research. Contact our experts today to find the perfect electrochemical solutions for your laboratory's specific needs.
Visual Guide
Related Products
- Gold Electrochemical Sheet Electrode Gold Electrode
- Gold Disc Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
People Also Ask
- How should a gold plate electrode be handled during an experiment? Ensure Accurate and Reproducible Results
- How should contamination of a gold plate electrode be prevented and managed? Essential Care for Reliable Data
- What are the disadvantages of gold electrodes? Key Limitations for Your Lab Projects
- What precautions should be taken to prevent mechanical damage to a gold plate electrode? Protect Your Data Integrity
- What are gold electrodes used for? Achieve Unmatched Sensitivity in Biosensing and Research



















