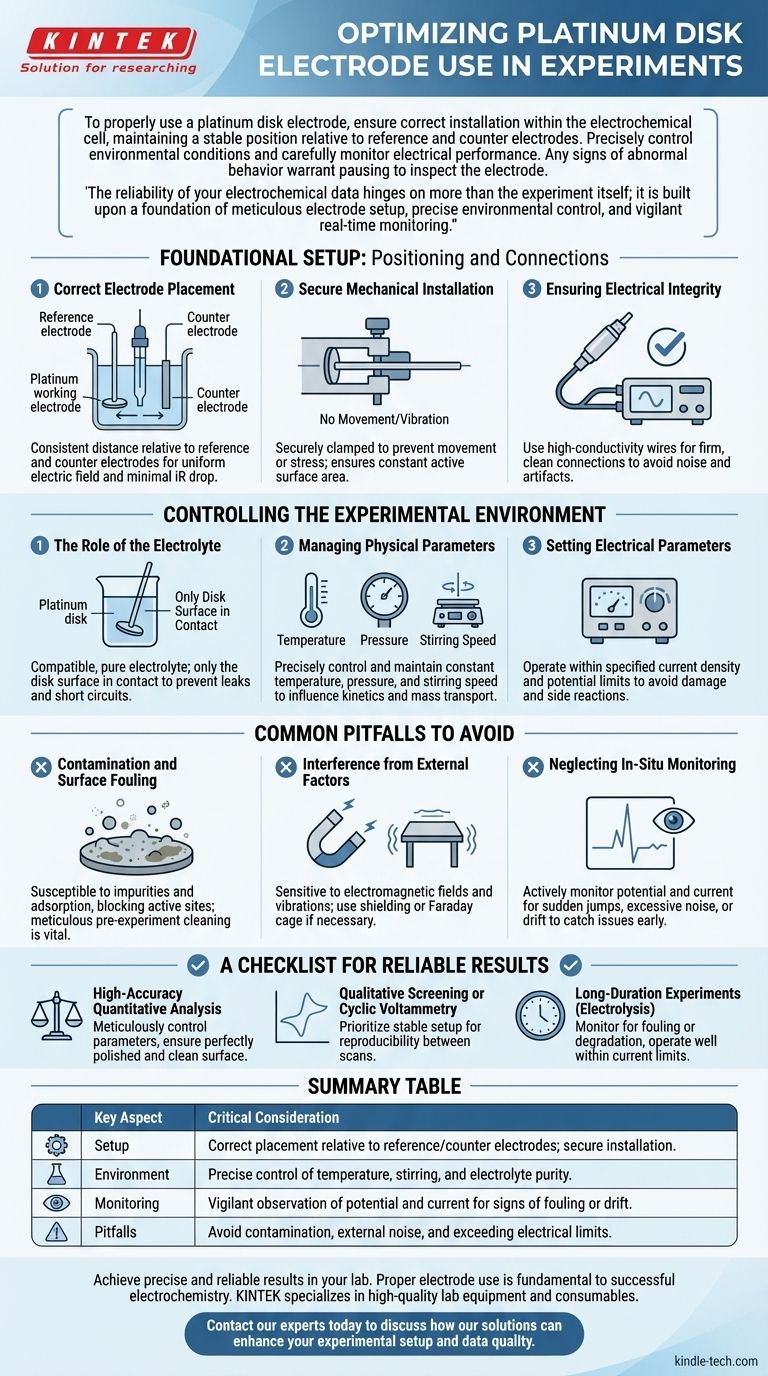
To properly use a platinum disk electrode, you must ensure it is correctly installed within the electrochemical cell, maintaining a stable position relative to the reference and counter electrodes. During the experiment, you need to precisely control environmental conditions like temperature and stirring speed while carefully monitoring the electrode's electrical performance. Any signs of abnormal behavior, such as a drifting potential, warrant pausing the experiment to inspect the electrode.
The reliability of your electrochemical data hinges on more than the experiment itself; it is built upon a foundation of meticulous electrode setup, precise environmental control, and vigilant real-time monitoring.

Foundational Setup: Positioning and Connections
Proper setup is the first step toward acquiring accurate and reproducible data. Errors made here will compromise every subsequent measurement.
Correct Electrode Placement
The platinum disk (the working electrode) must be positioned carefully relative to the reference electrode and the counter electrode.
A consistent and appropriate distance ensures a uniform electric field and minimizes uncompensated solution resistance (iR drop), a common source of error in electrochemical measurements.
Secure Mechanical Installation
The electrode must be securely clamped or held in the electrochemical cell to prevent any movement, vibration, or mechanical stress like bending.
A stable electrode maintains a constant active surface area exposed to the electrolyte, which is critical for quantitative analysis.
Ensuring Electrical Integrity
Use high-conductivity wires to connect the electrode to the potentiostat, ensuring a firm, clean connection.
A poor electrical contact can introduce significant noise and artifacts into your measurements, making the data difficult or impossible to interpret.
Controlling the Experimental Environment
The electrochemical system is highly sensitive to its surroundings. Controlling these variables is non-negotiable for achieving reliable results.
The Role of the Electrolyte
Select an electrolyte that is compatible with your reaction and will not corrode or react with the platinum. Ensure its purity and concentration meet your experimental requirements.
Crucially, only the platinum disk surface should be in contact with the electrolyte. Submerging other parts of the electrode body can lead to leaks, contamination, and short circuits.
Managing Physical Parameters
Experimental conditions such as temperature, pressure, and stirring speed must be precisely controlled and kept constant.
These factors directly influence reaction kinetics and mass transport (the rate at which reactants reach the electrode surface), and any fluctuation will affect your current and potential readings.
Setting Electrical Parameters
Operate within the electrode's specified limits for current density and potential.
Exceeding these limits can cause irreversible damage to the electrode surface, evolve gas, or trigger unwanted side reactions that interfere with your primary measurement.
Common Pitfalls to Avoid
Even with a perfect setup, several factors can compromise your experiment. Being aware of these common issues is critical for troubleshooting and ensuring data quality.
Contamination and Surface Fouling
The platinum surface is highly catalytic and susceptible to contamination from impurities in the electrolyte or adsorption of reaction products.
This fouling can block active sites on the electrode, leading to a decrease in current and a loss of electrochemical activity. This is why meticulous pre-experiment cleaning and polishing are so vital.
Interference from External Factors
Electrochemical measurements, which often involve very small currents, are sensitive to external noise.
Keep your setup away from sources of electromagnetic fields (e.g., power supplies, stir plates) and mechanical vibrations, as these can introduce noise and instability into your data. Use shielding or a Faraday cage if necessary.
Neglecting In-Situ Monitoring
Do not simply start the experiment and walk away. Actively monitor the electrode's performance by observing the potential and current readings.
Sudden jumps, excessive noise, or a steady drift can indicate problems like bubble formation on the surface, contamination, or a failing reference electrode. Catching these issues early can save the experiment.
A Checklist for Reliable Results
Your approach to using the electrode should align with the specific goals of your experiment.
- If your primary focus is high-accuracy quantitative analysis: Meticulously control all parameters, especially temperature and stirring, and ensure the electrode surface is perfectly polished and clean before each run.
- If your primary focus is qualitative screening or cyclic voltammetry: Prioritize a stable and secure setup to ensure reproducibility between scans and prevent mechanical noise from obscuring subtle electrochemical features.
- If your primary focus is long-duration experiments (e.g., electrolysis): Pay closest attention to monitoring for electrode fouling or electrolyte degradation and operate well within the electrode's current density limits to prevent damage.
By treating your electrode not as a simple component but as a precision instrument, you ensure the integrity and reliability of your electrochemical work.
Summary Table:
| Key Aspect | Critical Consideration |
|---|---|
| Setup | Correct placement relative to reference/counter electrodes; secure installation. |
| Environment | Precise control of temperature, stirring, and electrolyte purity. |
| Monitoring | Vigilant observation of potential and current for signs of fouling or drift. |
| Pitfalls | Avoid contamination, external noise, and exceeding electrical limits. |
Achieve precise and reliable results in your lab. Proper electrode use is fundamental to successful electrochemistry. KINTEK specializes in high-quality lab equipment and consumables, including electrodes and electrochemical cells, to support your research needs. Contact our experts today to discuss how our solutions can enhance your experimental setup and data quality.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Gold Disc Electrode
People Also Ask
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance
- What is the expected lifespan of a platinum sheet electrode? Maximize Your Electrode's Service Life
- What precautions should be taken when using a platinum sheet electrode? Ensure Accurate & Reproducible Electrochemical Data
- What is the proper post-treatment procedure for a platinum sheet electrode? Ensure Long-Term Accuracy & Protect Your Investment
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance



















