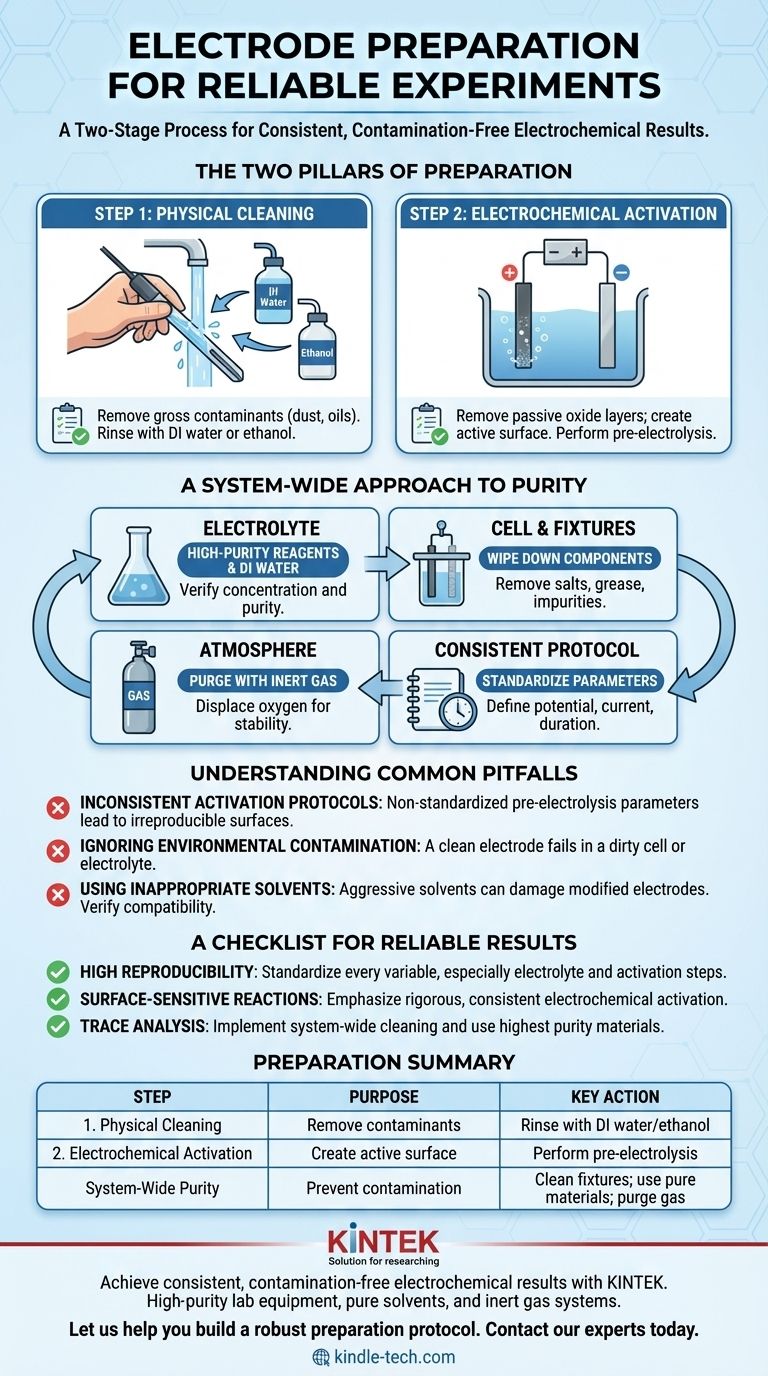
To properly prepare electrodes, you must execute a two-stage process. First, perform a physical cleaning of the electrode surface using a solvent like deionized water or ethanol to remove any gross impurities. Second, perform an electrochemical activation, often by pre-electrolysis in your electrolyte, to remove passive oxide layers and create a uniformly reactive surface for your experiment.
The core principle of electrode preparation is not just cleaning the electrode itself, but ensuring the entire electrochemical system—the electrode, electrolyte, and cell—is free of contaminants and that the electrode surface is in a known, active state before you begin.

The Two Pillars of Electrode Preparation
A reliable electrochemical measurement depends entirely on the state of the electrode surface. The goal is to make that surface both clean and electrochemically active in a reproducible way. This is achieved through two distinct but equally important steps.
Step 1: Physical Cleaning
This initial step is about removing any external contaminants that may have accumulated on the electrode during storage or handling.
Common contaminants include dust, oils from handling, or residues from previous experiments. A simple wipe or rinse with a high-purity solvent is typically sufficient.
Deionized (DI) water is a universal solvent for salts and polar residues. Ethanol is effective for removing organic films and grease. The choice depends on the likely contaminants and the electrode material's chemical resistance.
Step 2: Electrochemical Activation
Physical cleaning is not enough. Most metal electrodes form a thin, passive, and non-conductive oxide layer when exposed to air. Activation removes this layer and prepares the surface for electron transfer.
This is most often done via pre-electrolysis. By running a current through the cell for a short period before the main experiment, you can electrochemically reduce the surface oxides, creating a fresh, highly active metallic surface.
The exact potential and duration of activation should be determined for your specific system and, crucially, kept consistent across all experiments to ensure reproducibility.
A System-Wide Approach to Purity
A perfectly prepared electrode will fail in a contaminated environment. True experimental control requires you to consider every component that interacts with your electrochemical reaction.
The Electrolyte: Purity is Paramount
Your electrolyte is an active participant in the reaction. Impurities can introduce unwanted side reactions, poison your electrode surface, or alter the very process you intend to measure.
Always use high-purity chemical reagents and deionized or distilled water. The concentration and purity should be verified to meet your experimental standards.
The Cell and Fixtures: Preventing Cross-Contamination
Contamination doesn't just come from chemicals. The physical apparatus is a common source of error.
Before assembly, wipe down all components—electrode clamps, the electrolytic cell body, and any fixtures—with deionized water or alcohol. This removes residual salts, grease, or other impurities that could leach into your electrolyte.
Controlling the Atmosphere
Many electrochemical reactions are highly sensitive to oxygen. Atmospheric oxygen can dissolve in the electrolyte and act as an unwanted oxidizing agent, creating significant interference.
If your experiment is sensitive to air, purge the cell with an inert gas like nitrogen or argon before and during the experiment. This displaces the oxygen and provides a stable, non-reactive environment.
Understanding Common Pitfalls
Meticulous preparation prevents failed experiments. Being aware of common mistakes is essential for developing a robust protocol.
Inconsistent Activation Protocols
The term "a short period" of pre-electrolysis is insufficient for reproducible science. The potential, current density, and duration of the activation step directly influence the final state of the electrode surface. These parameters must be standardized.
Ignoring Environmental Contamination
The most common failure is focusing solely on the electrode while ignoring the cell and electrolyte. A perfectly activated electrode placed into an electrolyte contaminated by dirty glassware or fixtures will produce meaningless data.
Using Inappropriate Solvents
While ethanol and DI water are common, they are not universally safe. Some modified electrodes, binders, or polymer coatings can be damaged by aggressive solvents. Always verify solvent compatibility with your specific electrode material.
A Checklist for Reliable Results
Your preparation strategy should align with your primary experimental goal. Use these points as a guide to building your standard operating procedure.
- If your primary focus is high reproducibility: Standardize every variable, especially the electrolyte batch, cleaning procedure, and the exact potential and duration of electrochemical activation.
- If your primary focus is studying surface-sensitive reactions: Emphasize rigorous electrochemical activation to ensure you begin every experiment with a known and identical surface state.
- If your primary focus is preventing contamination in trace analysis: Implement system-wide cleaning of the cell and fixtures, and use the highest purity electrolyte and solvents available.
This diligent preparation is the foundation upon which all trustworthy electrochemical data is built.
Summary Table:
| Step | Purpose | Key Action |
|---|---|---|
| 1. Physical Cleaning | Remove gross contaminants (dust, oils) | Rinse with DI water or ethanol |
| 2. Electrochemical Activation | Remove passive oxide layers; create active surface | Perform pre-electrolysis in electrolyte |
| System-Wide Purity | Prevent contamination from cell/environment | Clean fixtures; use pure electrolyte; purge with inert gas if needed |
Achieve consistent, contamination-free electrochemical results with KINTEK.
Proper electrode preparation is critical for reliable data. KINTEK specializes in high-purity lab equipment and consumables—including electrochemical cells, pure solvents, and inert gas systems—to support your precise experimental needs.
Let us help you build a robust preparation protocol. Contact our experts today to discuss your lab's specific requirements and ensure your experiments start on a solid foundation.
Visual Guide
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Glassy Carbon Electrochemical Electrode
- Copper Sulfate Reference Electrode for Laboratory Use
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Gold Disc Electrode
People Also Ask
- Which type of electrode can be used as a reference point? Select the Right One for Accurate Measurements
- What is the reference electrode for mercury mercury chloride? Discover the Saturated Calomel Electrode (SCE)
- What are the four main types of sensors? A Guide to Power Source and Signal Type
- What is the reference electrode for mercury mercurous sulfate? A Guide to Chloride-Free Electrochemistry
- What are the characteristics of a saturated calomel electrode for neutral solutions? Understanding its stability and limitations.



















