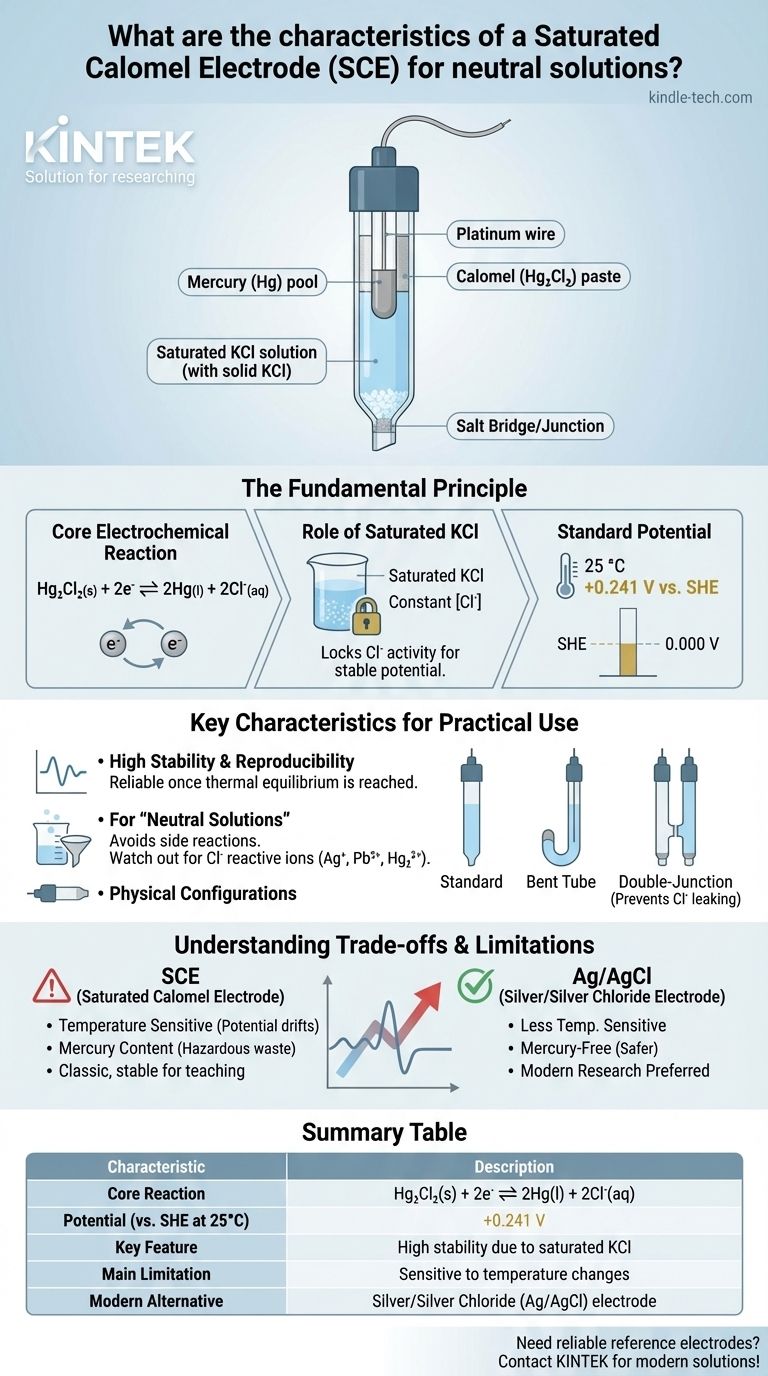
At its core, a Saturated Calomel Electrode (SCE) for neutral solutions is a reference electrode that provides a stable and well-defined electrical potential. Its defining characteristic is the use of a paste of mercury and mercury(I) chloride (calomel) in contact with a solution saturated with potassium chloride (KCl), which acts as the salt bridge. This specific chemical composition is what allows it to serve as a reliable benchmark for electrochemical measurements.
The Saturated Calomel Electrode is a classic, highly stable reference electrode valued for its reproducible potential in general-purpose applications. However, its performance is sensitive to temperature changes, and the presence of mercury makes modern alternatives like the Silver/Silver Chloride electrode often preferable.

The Fundamental Principle: How an SCE Works
To trust an electrode, you must first understand its inner workings. The SCE's stability is not magic; it's a direct result of a carefully controlled chemical equilibrium.
The Core Electrochemical Reaction
The potential of the SCE is generated by a reversible redox reaction involving mercury and its sparingly soluble salt, calomel (Hg₂Cl₂).
The half-reaction is: Hg₂Cl₂(s) + 2e⁻ ⇌ 2Hg(l) + 2Cl⁻(aq)
This equilibrium establishes a specific potential that is dependent on the concentration (more accurately, the activity) of the chloride ions (Cl⁻) in the solution.
The Role of Saturated KCl
This is the key to the electrode's stability. By using a saturated solution of potassium chloride, the concentration of chloride ions is kept constant and at a maximum.
As long as solid KCl crystals are present, the solution remains saturated, effectively "locking" the chloride ion activity. This constant activity ensures the electrode's potential remains stable and does not drift during an experiment.
The Standard Potential
Because of this stable equilibrium, the SCE has a well-known potential relative to the Standard Hydrogen Electrode (SHE), which is the universal baseline for electrochemistry.
At 25 °C, the potential of a Saturated Calomel Electrode is approximately +0.241 Volts versus SHE. This known value is what allows you to measure the potential of your working electrode against a reliable constant.
Key Characteristics for Practical Use
Understanding the theory is one thing; applying it in the lab is another. The design of the SCE directly reflects its intended use.
High Stability and Reproducibility
The primary reason for the SCE's long history of use is its excellent potential stability. Once thermal equilibrium is reached, its potential is highly reproducible, making it a reliable standard for precise measurements in controlled environments.
The "Neutral Solution" Constraint
The SCE is specified for "neutral solutions" primarily to avoid unwanted side reactions. The main concern is the KCl salt bridge leaking chloride ions into the sample.
If your sample contains ions that form insoluble precipitates with chloride, such as silver (Ag⁺), lead (Pb²⁺), or mercury(I) (Hg₂²⁺), the junction can become clogged, leading to unstable readings.
Physical Configurations
Commercial SCEs are available in various forms to suit different experimental setups.
- Standard and Extended versions are for general-purpose use in beakers.
- Bent tube versions are designed for smaller or uniquely shaped electrochemical cells.
- Double-Junction versions are the solution to the chloride precipitation problem. They have an outer chamber filled with a non-interfering electrolyte (like potassium nitrate), which prevents the KCl from directly contacting the sample.
Understanding the Trade-offs and Limitations
No instrument is perfect. A true expert understands not only when to use a tool, but also when not to.
Temperature Sensitivity
This is the SCE's most significant practical weakness. The solubility of KCl changes dramatically with temperature. As the temperature fluctuates, the chloride ion concentration changes, which in turn alters the electrode's potential. For this reason, SCEs are not ideal for experiments with significant temperature variations.
Mercury Content
The use of mercury is a major environmental and health hazard. Due to regulations and safety concerns, many labs have phased out calomel electrodes in favor of mercury-free alternatives. Disposing of old or broken SCEs requires special hazardous waste procedures.
The Rise of the Ag/AgCl Electrode
The Silver/Silver Chloride (Ag/AgCl) electrode has largely replaced the SCE as the reference electrode of choice. It operates on a similar principle but avoids the use of mercury and exhibits less temperature sensitivity, making it a more robust and safer alternative for most applications.
Making the Right Choice for Your Goal
Selecting the correct reference electrode is critical for accurate data. Your choice depends entirely on your experimental priorities and constraints.
- If your primary focus is teaching or replicating classic experiments: The SCE is a historically significant electrode that provides excellent stability in a temperature-controlled lab.
- If your primary focus is general-purpose modern research: A Silver/Silver Chloride (Ag/AgCl) electrode is almost always the better choice due to its safety, lower temperature sensitivity, and comparable stability.
- If your primary focus is analyzing samples with chloride-reactive ions (like Ag⁺): You must use a double-junction reference electrode, regardless of whether it is an SCE or Ag/AgCl model.
Ultimately, understanding the principles of how each reference electrode functions empowers you to select the right tool for the job.
Summary Table:
| Characteristic | Description |
|---|---|
| Core Reaction | Hg₂Cl₂(s) + 2e⁻ ⇌ 2Hg(l) + 2Cl⁻(aq) |
| Potential (vs. SHE at 25°C) | +0.241 V |
| Key Feature | High stability due to saturated KCl |
| Main Limitation | Sensitive to temperature changes |
| Modern Alternative | Silver/Silver Chloride (Ag/AgCl) electrode |
Need a reliable reference electrode for your lab? KINTEK specializes in lab equipment and consumables, offering a wide range of electrochemical tools, including modern alternatives like Ag/AgCl electrodes. Our experts can help you select the right equipment for precise, safe, and efficient measurements. Contact us today to enhance your laboratory's capabilities!
Visual Guide
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Gold Disc Electrode
- Glassy Carbon Electrochemical Electrode
People Also Ask
- What is the reference electrode for mercury mercury chloride? Discover the Saturated Calomel Electrode (SCE)
- Which electrode is used as a reference? A Guide to Accurate Electrochemical Measurements
- What are the four main types of sensors? A Guide to Power Source and Signal Type
- Why is a Saturated Calomel Electrode (SCE) used as a reference electrode in microbial fuel cell research?
- Why and how should the electrodes of an electrolytic cell be calibrated? Ensure Reliable Results



















