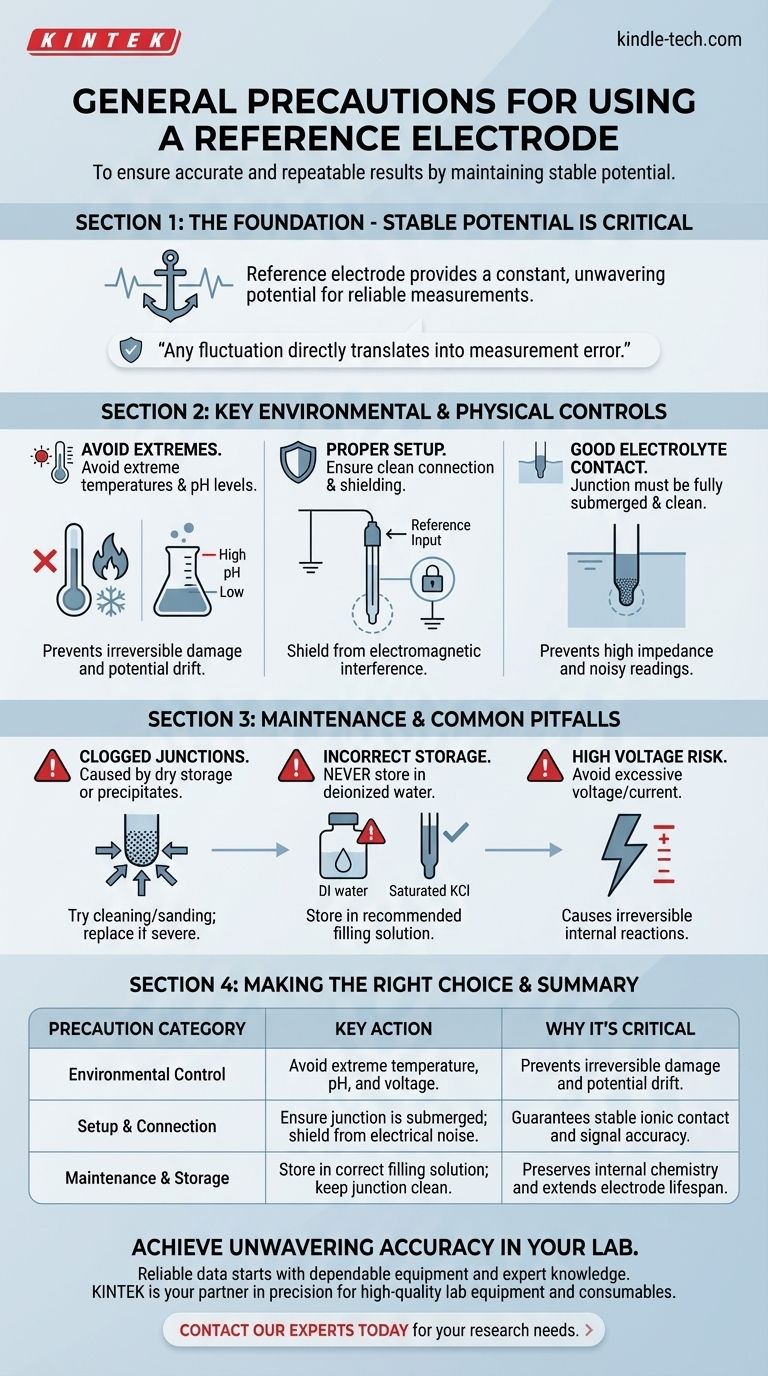
To ensure accurate and repeatable results, the general precautions for using a reference electrode center on maintaining its stable potential. This is achieved by controlling its environment, ensuring proper electrical and physical contact, and protecting it from external interference. You must avoid extreme temperatures, voltages, and pH levels, as these can irreversibly damage the electrode or cause its potential to drift, invalidating your experiment.
The core purpose of a reference electrode is to provide a constant, unwavering potential. Every precaution is designed to protect this stability, as any fluctuation in the reference electrode directly translates into error in your measurement.

The Foundation: Why Reference Electrode Stability is Critical
A reference electrode is the stable anchor in any electrochemical measurement. The potential of your working electrode is always measured relative to this reference.
If the reference electrode's potential shifts, your entire measurement shifts with it, making your data unreliable. Protecting its stability is your primary responsibility.
The Impact of Temperature
High temperatures can alter the concentration of the internal filling solution and change the equilibrium of the electrode's internal chemical reaction.
This directly causes the reference potential to drift, introducing a systematic error into all your measurements. It is always best to conduct experiments at a stable, known temperature.
The Danger of Extreme pH
Highly acidic or alkaline solutions can attack the porous junction (or frit) that allows ionic contact between the electrode and your sample.
This can damage the electrode, cause it to clog, or allow your sample to contaminate the internal filling solution, all of which lead to potential drift and inaccurate readings.
Proper Setup for Reliable Data
How you physically and electrically connect the electrode is just as important as the chemical environment. Errors in setup are a common source of failed experiments.
Ensuring a Clean Electrical Connection
Connect the electrode's lead wire to the dedicated reference electrode input on your potentiostat.
To ensure signal accuracy, shield the electrode and its cabling from electromagnetic interference (e.g., power lines, monitors, motors). Using a grounded Faraday cage is a best practice for low-current measurements.
Guaranteeing Good Electrolyte Contact
The porous junction or frit at the tip of the electrode must be fully submerged in your sample electrolyte.
This junction must also be clean and unclogged. A clogged junction creates high impedance, leading to noisy, unstable, or completely incorrect readings.
Maintaining the Filling Solution
The internal filling solution must be at the correct concentration and free of contamination.
The level of the filling solution should also be higher than the level of the sample solution. This creates positive hydrostatic pressure, ensuring a slow outflow that prevents your sample from contaminating the electrode's internal components.
Common Pitfalls and How to Avoid Them
Even with a proper setup, poor handling and storage practices can degrade electrode performance and shorten its lifespan.
The Problem of Clogged Junctions
Junctions can become clogged if the electrode is stored dry or if the filling solution reacts with the sample to form a precipitate.
If you suspect a clog, you can try cleaning it or, for some models, carefully sanding the frit. However, a severe clog often means the electrode must be replaced.
The Mistake of Incorrect Storage
Never store a reference electrode in deionized water, as this will cause the filling solution to leach out, changing its potential.
Always store the electrode in its recommended filling solution (e.g., saturated KCl for a standard Ag/AgCl electrode). This keeps the internal chemistry stable and ready for the next measurement.
The Risk of High Voltage
Applying an excessively high voltage or current across the reference electrode can cause irreversible electrochemical reactions inside it.
This will permanently damage the electrode and destroy its stable potential. Always operate within the voltage limits specified by your potentiostat and experimental design.
Making the Right Choice for Your Goal
Your primary goal dictates which precautions are most critical in the moment.
- If your primary focus is measurement accuracy: Double-check that the junction is clean and fully submerged, and shield your setup from electrical noise with a Faraday cage.
- If your primary focus is electrode longevity: Always store the electrode in its correct filling solution and be diligent about avoiding extreme temperatures and pH.
- If you are troubleshooting unstable readings: The most likely culprits are a clogged junction, a contaminated filling solution, or electrical noise in the environment.
Treating your reference electrode with care is the first and most critical step toward generating reliable and reproducible electrochemical data.
Summary Table:
| Precaution Category | Key Action | Why It's Critical |
|---|---|---|
| Environmental Control | Avoid extreme temperature, pH, and voltage. | Prevents irreversible damage and potential drift. |
| Setup & Connection | Ensure junction is submerged; shield from electrical noise. | Guarantees stable ionic contact and signal accuracy. |
| Maintenance & Storage | Store in correct filling solution; keep junction clean. | Preserves internal chemistry and extends electrode lifespan. |
Achieve Unwavering Accuracy in Your Lab
Generating reliable electrochemical data starts with dependable equipment and expert knowledge. The precautions outlined above are essential for protecting your reference electrodes and ensuring measurement integrity.
KINTEK is your partner in precision. We specialize in high-quality lab equipment and consumables, serving the exacting needs of laboratories like yours. Whether you are setting up a new electrochemical station or need trusted consumables for daily use, our products are designed for performance and longevity.
Let us help you enhance your lab's capabilities. Contact our experts today to discuss your specific requirements and discover how KINTEK can support your research goals.
Visual Guide
Related Products
- Copper Sulfate Reference Electrode for Laboratory Use
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Metal Disc Electrode Electrochemical Electrode
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
People Also Ask
- Is there a difference in performance between wood plug and ceramic core copper sulfate electrodes? Speed vs. Durability Explained
- What are the pre-treatment steps before using a portable copper sulfate reference electrode? Ensure Accurate Corrosion Potential Measurements
- How should a copper sulfate reference electrode be stored? A Guide to Short-Term & Long-Term Storage
- What are the advantages and disadvantages of the wood plug type copper sulfate reference electrode? Speed vs. Durability Explained
- What are the post-treatment procedures after using a copper sulfate reference electrode? Essential Steps for Accuracy & Longevity



















