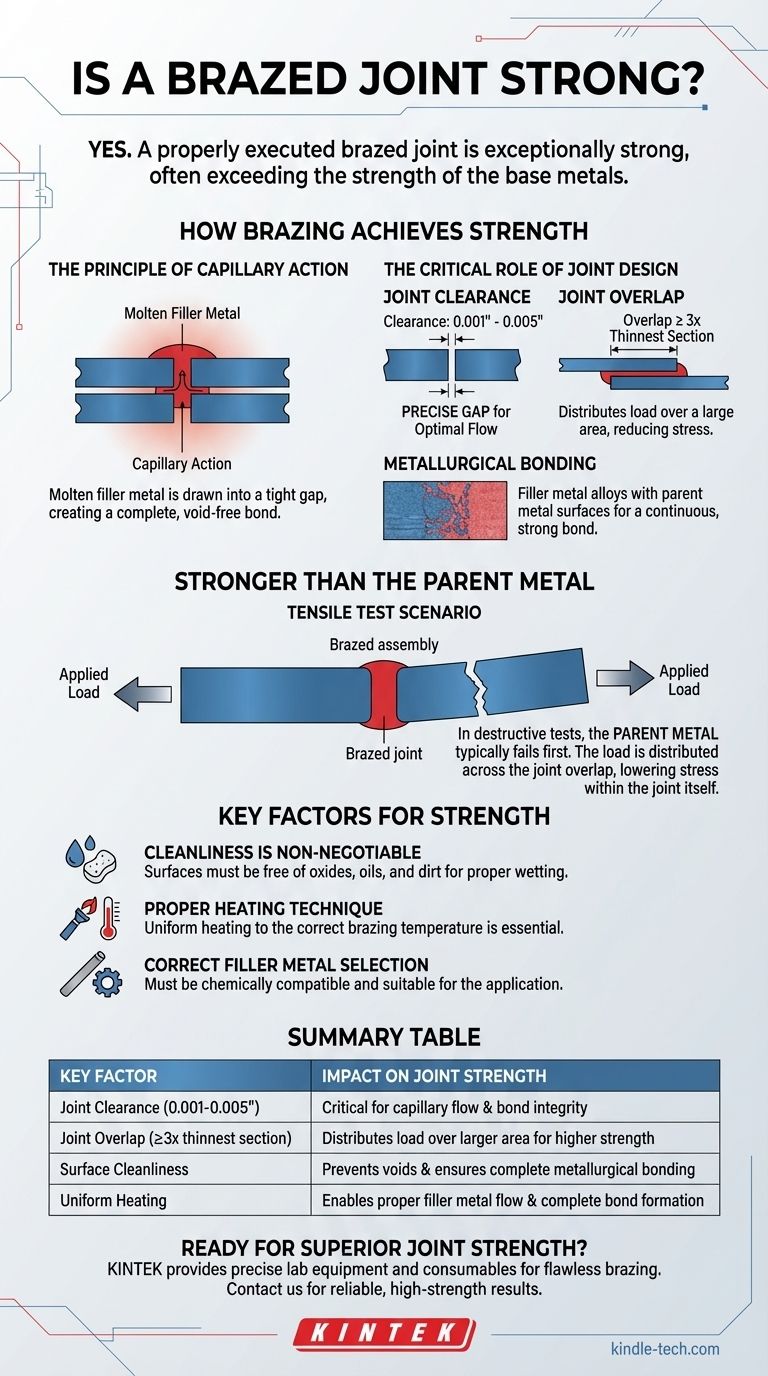
Yes, a properly executed brazed joint is exceptionally strong. In fact, when a joint is correctly designed, prepared, and heated, its strength will typically exceed that of the individual base metals being joined. This means that in a destructive test, the base metal will often fail before the brazed joint itself gives way.
The strength of a brazed joint is not a default outcome; it is the result of proper engineering. The process relies on a phenomenon called capillary action to create a complete, high-strength bond over a large surface area, making the joint's design as critical as the brazing process itself.
How Brazing Achieves Its Strength
The remarkable strength of a brazed joint comes from the interplay between the joint's design, the filler metal, and the base metals. It is not simply a matter of melting a filler rod between two pieces of metal.
The Principle of Capillary Action
Brazing works by heating the base metals (the "parent metals") to a temperature above the melting point of the brazing filler metal.
The molten filler metal is then drawn into the tight space between the two closely fitted surfaces via capillary action. This natural force ensures the filler metal is distributed evenly, creating a complete and void-free bond.
The Critical Role of Joint Design
Unlike welding, brazing's strength is directly proportional to the surface area of the bond. Two factors are paramount.
Joint Clearance: The gap between the two parent metals must be precise. If the gap is too wide, capillary action will be weak or nonexistent. If it's too tight, the filler metal cannot flow into the joint at all. For most common filler metals, this gap is typically between 0.001" and 0.005" (0.025 mm to 0.127 mm).
Joint Overlap: The strength of the joint is a function of its shear area. A good rule of thumb is to design the joint with an overlap of at least three times the thickness of the thinnest metal section. This overlap distributes the load over a large area, reducing stress on any single point.
The Power of Metallurgical Bonding
The filler metal doesn't just act like glue. During the brazing process, the filler metal alloys with a thin layer of the parent metal surfaces. This creates a new, strong, and continuous metallurgical bond between the parts.
Understanding the "Stronger Than the Parent Metal" Phenomenon
The statement that a brazed joint can be stronger than the metals it joins is consistently proven in lab tests and real-world applications.
The Tensile Test Scenario
When a properly brazed assembly is put under a tensile (pull-apart) test, failure rarely occurs at the brazed joint itself.
Instead, the parent metal next to the joint will typically stretch, deform, and break first. The joint remains intact.
Why This Happens
The load applied to the assembly is distributed across the entire surface area of the joint overlap. Because this area is significant (due to the 3T overlap rule), the stress within the joint is lower than the stress in the narrower cross-section of the parent metal.
Essentially, you are pulling on a wide, strong bond, and the weakest link becomes the base material itself.
Key Factors That Determine Strength
Achieving this level of strength is conditional. The final outcome depends entirely on the process.
Cleanliness is Non-Negotiable
The surfaces of the parent metals must be scrupulously clean. Any oxides, oils, or dirt will prevent the filler metal from wetting the surface and flowing properly, resulting in voids and a dramatically weakened joint. The use of a proper flux or a controlled atmosphere is essential to prevent oxidation during heating.
Proper Heating Technique
Both parent metals must be heated uniformly to the correct brazing temperature. If one part is hotter than the other, the filler metal will only flow toward the hotter section, creating an incomplete bond.
Correct Filler Metal Selection
The filler metal must be chemically compatible with the parent metals and suitable for the end-use application, considering factors like service temperature and potential for galvanic corrosion.
Making the Right Choice for Your Application
Use these guidelines to determine if brazing is the correct approach for your project.
- If your primary focus is joining dissimilar metals or delicate, thin-walled parts: Brazing is an excellent choice due to its lower process temperature, which minimizes distortion and thermal stress.
- If your primary focus is maximum strength in a joint that can be properly designed: A correctly executed brazed joint provides exceptional strength that rivals or exceeds the base materials.
- If you need to fill large, inconsistent gaps or require performance at extreme temperatures: Welding is likely a more suitable process for your needs, as brazing relies on tight clearances and its strength degrades as temperatures approach the filler metal's melting point.
Ultimately, brazing provides a robust and reliable joining method when the principles of joint design and process control are respected.
Summary Table:
| Key Factor | Impact on Joint Strength |
|---|---|
| Joint Clearance (0.001-0.005") | Critical for capillary flow and bond integrity |
| Joint Overlap (≥3x thinnest section) | Distributes load over larger area for higher strength |
| Surface Cleanliness | Prevents voids and ensures complete metallurgical bonding |
| Uniform Heating | Enables proper filler metal flow and complete bond formation |
Ready to achieve superior joint strength in your laboratory applications?
At KINTEK, we specialize in providing the precise lab equipment and consumables needed for flawless brazing processes. Our expertise ensures you get the right tools for proper joint design, temperature control, and surface preparation—helping you create brazed joints that consistently outperform the base materials.
Contact us today to discuss how our solutions can enhance your joining applications and deliver reliable, high-strength results. Get in touch with our experts to get started!
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Dental Porcelain Zirconia Sintering Ceramic Vacuum Press Furnace
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What is the function of high-temperature furnaces in HPQ processing? Optimize Quartz Calcination & Quenching
- What reaction conditions are provided by a high-temperature vacuum furnace during RMI? Optimize UHTCMC Manufacturing
- What is the vacuum heat treatment cycle? Achieve Superior Material Purity and Precision
- What is the body structure of a furnace? Unlocking the Dual-Layer Design for Superior Thermal Control
- How does a sintering furnace work? Achieve Superior Material Strength and Density
- Why is it necessary for a high-temperature furnace to maintain a constant 750°C for Sc1/3Zr2(PO4)3 DC electrolysis?
- Does metal evaporate in a vacuum? Unlock the Power of Thin-Film Deposition
- How does a sintering machine work? A Guide to Powder Metallurgy & Ceramic Fabrication



















