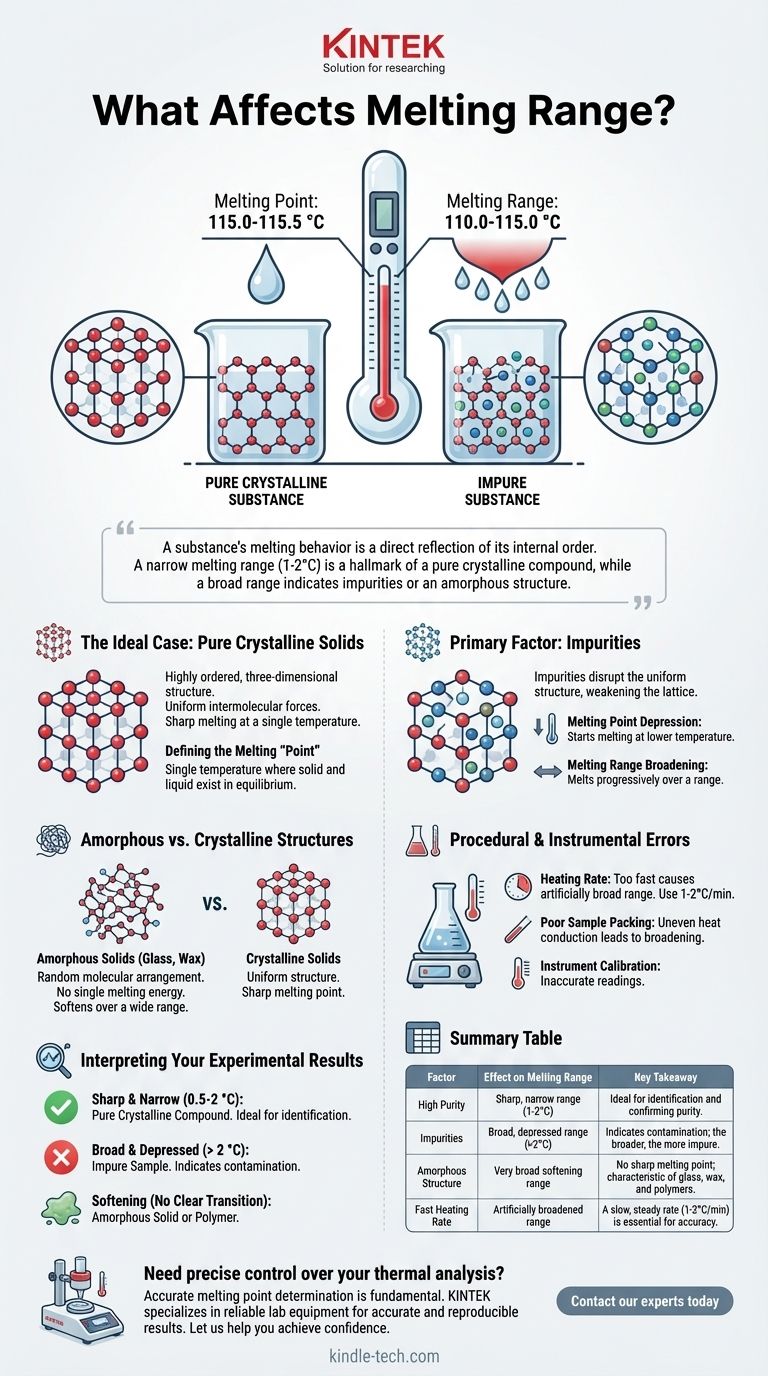
In chemistry, the single greatest factor that affects a substance's melting range is its purity. While a perfectly pure crystalline substance melts at a single, sharp temperature, the presence of impurities disrupts its uniform structure, causing it to melt gradually over a range of temperatures.
A substance's melting behavior is a direct reflection of its internal order. A narrow melting range (typically 1-2°C) is a hallmark of a pure crystalline compound, while a broad melting range indicates the presence of impurities or a non-crystalline (amorphous) structure.

The Ideal Case: Why Pure Solids Have a Sharp Melting Point
To understand what broadens a melting range, we must first understand the ideal scenario: a pure, crystalline solid.
The Role of the Crystal Lattice
A crystalline solid is defined by its highly ordered, three-dimensional structure, known as a crystal lattice. In this lattice, every molecule is locked in a specific position with uniform intermolecular forces holding it to its neighbors.
Melting is the process of providing enough thermal energy to overcome these forces and break down the lattice structure. Because the structure is so uniform, the energy required to break the bonds is consistent throughout the entire crystal.
Defining the Melting "Point"
The true melting point is the single temperature at which the solid and liquid phases of a pure substance exist in equilibrium. As you add heat, the temperature of the substance rises until it hits this point, where it stays constant until all the solid has transformed into a liquid.
Key Factors That Broaden the Melting Range
When a substance melts over a range instead of at a point, it's because this ideal uniformity has been compromised.
The Primary Factor: Impurities
Impurities are foreign particles mixed into the main compound. They disrupt the repeating pattern of the crystal lattice, weakening its overall structure.
This disruption has two key effects:
- Melting Point Depression: The weakened lattice requires less energy to begin breaking down. This means the substance will start to melt at a temperature lower than the pure compound.
- Melting Range Broadening: The impurities are not distributed perfectly evenly. Pockets of the substance with fewer impurities will require more energy to melt than areas rich in impurities. This causes melting to occur progressively over a range of temperatures.
Amorphous vs. Crystalline Structures
Not all solids are crystalline. Amorphous solids, such as glass, wax, and many polymers, lack a long-range ordered crystal lattice. Their molecules are arranged randomly, like a tangled ball of yarn.
Because there is no uniform structure, there is no single energy value required for melting. Different bonds have different strengths, so as heat is applied, the solid simply softens gradually over a wide temperature range. Amorphous solids don't have a sharp melting point; they have a "glass transition temperature" and a softening range.
Procedural and Instrumental Errors
Even a pure sample can appear to have a broad melting range due to poor lab technique.
- Heating Rate: Heating the sample too quickly in a melting point apparatus is the most common error. The thermometer cannot keep up with the actual temperature of the sample, causing you to record a wider range than is real. A slow, steady rate (1-2°C per minute) is critical for accuracy.
- Poor Sample Packing: A loosely packed sample in a capillary tube will not conduct heat uniformly, leading to an inaccurate and broadened melting range.
- Instrument Calibration: An uncalibrated thermometer will give you an inaccurate reading, making it impossible to compare your result to a known literature value for identification.
Interpreting Your Experimental Results
The characteristics of the melting range are a powerful diagnostic tool for a chemist.
Sharp and Narrow Range (e.g., 0.5-2 °C)
This is the gold standard for a pure crystalline compound. The narrowness of the range indicates high purity, and the temperature at which it melts can be used to identify the compound by comparing it to known values.
Broad and Depressed Range (e.g., > 2 °C)
This is the classic signature of an impure sample. The range begins at a temperature lower than the literature melting point of the pure substance and extends over several degrees. The broader the range, the more impure the sample is likely to be.
Softening Without a Clear Transition
If the substance simply gets softer, shrinks, and turns to goo over a very wide temperature range without ever becoming a clear liquid, you are likely dealing with an amorphous solid or a polymer.
How to Use Melting Range as a Diagnostic Tool
After measuring a melting range, you can use the data to draw specific conclusions.
- If your primary focus is assessing purity: The width of the melting range is your most important piece of data; a narrow range of 1-2°C is the goal for a pure crystalline compound.
- If your primary focus is identifying an unknown compound: A sharp, narrow melting range that matches a known literature value is strong evidence for identification, which can be confirmed using a mixed melting point test.
- If your primary focus is characterizing a polymer or mixture: Carefully document the temperature at which the substance begins to soften, the range over which it melts, and the temperature at which it becomes a fully transparent liquid.
Ultimately, observing a substance's melting behavior is one of the simplest yet most informative techniques available in the laboratory.
Summary Table:
| Factor | Effect on Melting Range | Key Takeaway |
|---|---|---|
| High Purity | Sharp, narrow range (1-2°C) | Ideal for identification and confirming purity. |
| Impurities | Broad, depressed range (>2°C) | Indicates contamination; the broader the range, the greater the impurity. |
| Amorphous Structure | Very broad softening range | No sharp melting point; characteristic of glass, wax, and polymers. |
| Fast Heating Rate | Artificially broadened range | A slow, steady rate (1-2°C/min) is essential for accuracy. |
Need precise control over your thermal analysis?
Accurate melting point determination is fundamental to chemical analysis and quality control. KINTEK specializes in providing reliable lab equipment, including precision melting point apparatuses and consumables, to ensure your results are accurate and reproducible. Whether you are purifying compounds, identifying unknowns, or characterizing materials, the right equipment is key.
Let us help you achieve confidence in your results. Contact our experts today to find the perfect solution for your laboratory's needs.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the uses of muffle furnace in pharmaceutical industry? Essential for Drug Purity & Safety
- How is a muffle furnace used for sample digestion? A Guide to Dry Ashing for Accurate Analysis
- How do you run a muffle furnace? Master the Step-by-Step Process for Safe, Precise Results
- How do you set up a muffle furnace? A Step-by-Step Guide for Safe and Accurate Operation
- What are the safety precautions for heat treatment? A Complete Guide to Protecting Personnel and Facilities



















