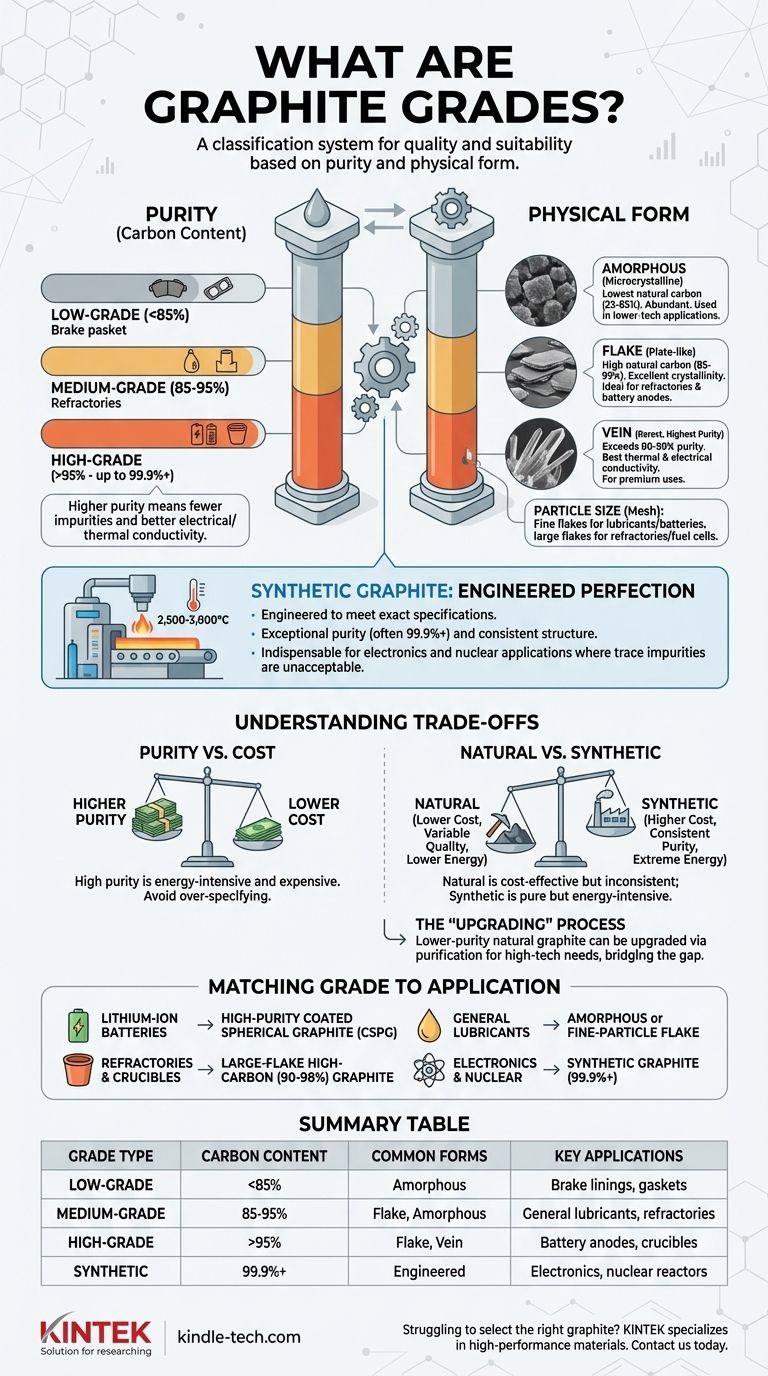
Graphite grades are a classification system used to define the quality and suitability of graphite for specific industrial purposes. These grades are primarily determined by two key factors: purity, measured as carbon content, and physical form, which includes the material's crystallinity and particle size. This system allows engineers and manufacturers to select the precise type of graphite that meets the performance and cost requirements of their application.
Choosing the right graphite is not about finding the "best" grade, but about matching the material's specific properties to the demands of your project. The critical decision hinges on balancing the required purity (carbon content) and physical form against the total cost.

The Two Pillars of Graphite Grading: Purity and Form
Graphite's value and function are almost entirely dictated by its purity and its physical structure. These two characteristics are the foundation of all grading systems.
Carbon Content: The Primary Metric of Purity
The most common way to grade graphite is by its carbon content. The higher the percentage of carbon, the fewer impurities (like ash, silica, or other minerals) are present.
- Low-Grade Graphite: Typically contains less than 85% carbon. It is often referred to as amorphous graphite.
- Medium-Grade Graphite: Ranges from 85% to 95% carbon.
- High-Grade Graphite: Contains over 95% carbon, with specialty grades for applications like batteries reaching 99.9% purity or higher.
Higher purity directly correlates with better electrical and thermal conductivity, making it essential for demanding applications.
Physical Form: The Structural Foundation
Natural graphite occurs in three distinct physical forms, each with unique properties and applications.
- Amorphous Graphite: Despite its name, this form is microcrystalline. It has the lowest natural carbon content (typically 25-85%) and is the most abundant. It's used in lower-tech applications like brake linings, gaskets, and foundry facings where high purity is not the main driver.
- Flake Graphite: This form consists of flat, plate-like particles. It has a much higher natural carbon content (85-99%) and excellent crystallinity. Its structure makes it ideal for refractories (resisting high heat) and, most critically, for the anodes in lithium-ion batteries after being processed into spherical graphite.
- Vein Graphite: This is the rarest and often highest-purity form of natural graphite, found in underground veins. With purities often exceeding 90-99%, it boasts the best thermal and electrical conductivity, making it a premium material for specialized lubricants and battery components.
The Role of Particle Size (Mesh)
Within each grade and form, particle size is a crucial secondary factor. Measured in "mesh," it dictates how the graphite will behave in a final product.
Large flakes (+50 mesh) are more valuable and sought after for applications like refractories and fuel cells. Finer flakes (-100 mesh) are used in lubricants, coatings, and battery anodes.
Synthetic Graphite: A Class of Its Own
Synthetic graphite is not mined but is an engineered product created by heating carbonaceous materials like petroleum coke to extremely high temperatures (2,500-3,000°C).
The Manufacturing Advantage
Because it is manufactured, synthetic graphite isn't graded by purity in the same way as natural graphite. Instead, it is engineered from the ground up to meet exact specifications.
Purity and Consistency by Design
The key benefit of synthetic graphite is its exceptional purity (often 99.9% or higher) and highly ordered, consistent crystal structure. This makes it indispensable for applications where even trace impurities are unacceptable, such as in nuclear reactors, semiconductor manufacturing, and electric motor brushes.
Understanding the Trade-offs
Selecting the right graphite grade requires a clear understanding of the compromises between performance, cost, and sourcing.
Purity vs. Cost
This is the fundamental trade-off. Increasing graphite purity is an energy-intensive and expensive process. A high-purity flake graphite (99.9%) can be many times more expensive than a standard grade (94%). Using a grade with higher purity than your application requires is a common and costly error.
Natural vs. Synthetic
Natural graphite is generally more cost-effective and has a significantly lower energy footprint during initial production. However, its quality can be inconsistent.
Synthetic graphite offers unparalleled purity and structural consistency but at a much higher financial and environmental cost due to the extreme energy required for its production.
The "Upgrading" Process
Lower-purity natural graphite is often "upgraded" to meet the demands of high-tech applications. This involves purification processes like flotation, chemical washing, or thermal treatment. This adds cost but transforms a lower-value raw material into a high-performance product, bridging the gap between natural supply and industrial demand.
Matching the Grade to Your Application
Use these guidelines to select the appropriate graphite based on your primary goal.
- If your primary focus is Lithium-Ion Batteries: You need high-purity (99.95%+) coated spherical graphite (CSPG), which is derived from high-grade flake graphite for optimal anode performance.
- If your primary focus is Refractories and Crucibles: You need large-flake, high-carbon (90-98%) graphite for its superior thermal shock resistance and non-wetting properties.
- If your primary focus is General-Purpose Lubricants: You can use lower-purity amorphous graphite for basic needs or fine-particle flake for higher-performance lubrication.
- If your primary focus is Absolute Purity for Electronics or Nuclear Uses: You must specify synthetic graphite for its engineered consistency and near-perfect carbon content.
Ultimately, understanding graphite grades is about moving beyond a simple "good vs. bad" mindset and embracing a precise, application-driven approach to material selection.
Summary Table:
| Grade Type | Carbon Content | Common Forms | Key Applications |
|---|---|---|---|
| Low-Grade | < 85% | Amorphous | Brake linings, gaskets |
| Medium-Grade | 85% - 95% | Flake, Amorphous | General lubricants, refractories |
| High-Grade | > 95% | Flake, Vein | Battery anodes, crucibles |
| Synthetic | 99.9%+ | Engineered | Electronics, nuclear reactors |
Struggling to select the right graphite for your lab or production needs? KINTEK specializes in high-performance lab equipment and consumables, including precision graphite materials for applications ranging from battery research to high-temperature processing. Our experts can help you match the perfect graphite grade to your specific requirements, ensuring optimal performance and cost-efficiency. Contact us today to discuss your project and discover how KINTEK's solutions can enhance your laboratory's capabilities!
Visual Guide
Related Products
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Horizontal High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Furnace Bottom Discharge Graphitization Furnace for Carbon Materials
- Large Vertical Graphite Vacuum Graphitization Furnace
People Also Ask
- What are the features and common uses of a graphite rod electrode? A Guide to Durable, Simple Electrochemistry
- How should a graphite electrode be cleaned and stored after an experiment? Ensure Reliable Electrochemical Data
- What are the properties and applications of a graphite disk electrode? Precision Tools for Electroanalysis
- Why is a high-purity graphite rod preferred as a counter electrode? Ensure Uncontaminated Electrochemical Analysis
- What are the potential risks when using a graphite electrode in electrochemical tests? Avoid Decomposition and Contamination



















