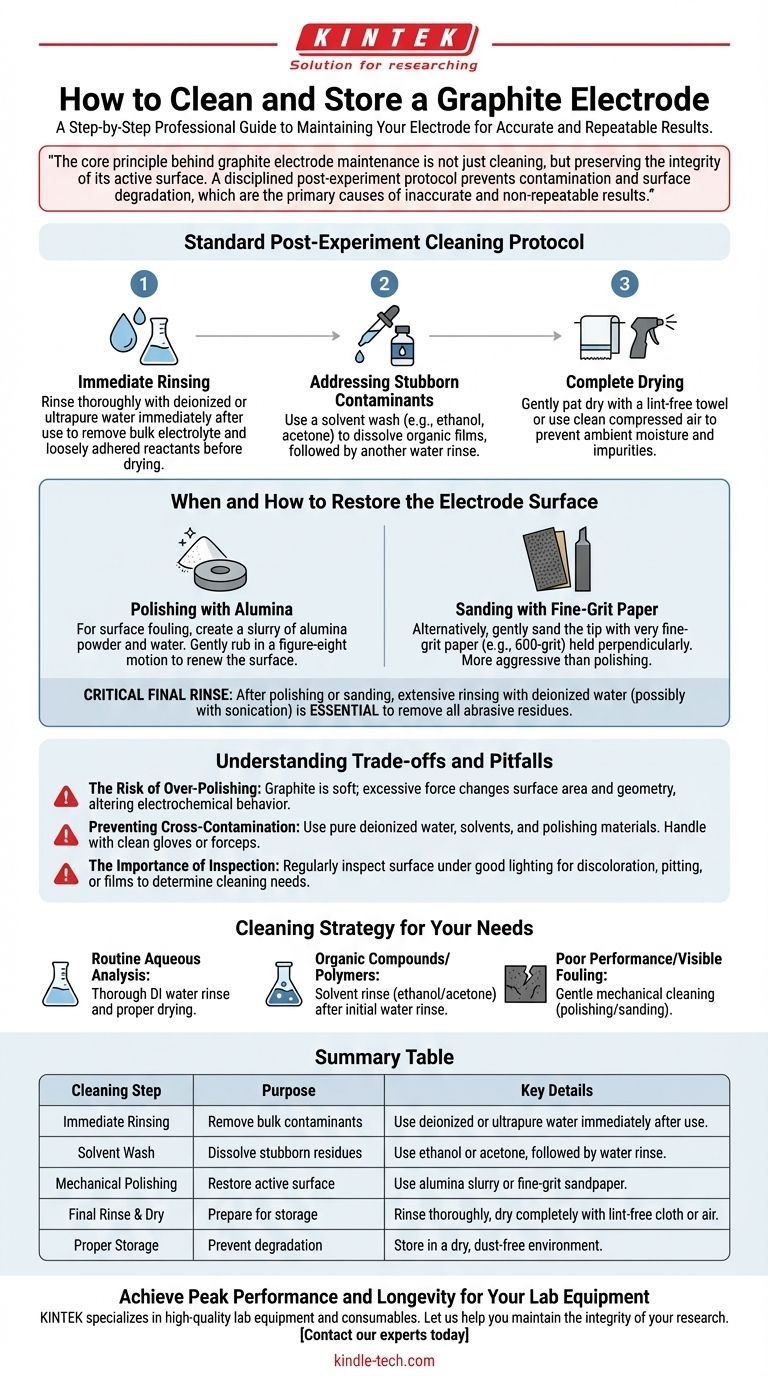
To properly clean and store a graphite electrode, you must perform a multi-step process immediately after use. Start by rinsing it thoroughly with deionized water to remove residual reactants, then use a solvent like ethanol for stubborn contaminants. If the surface is fouled, gently polish it with alumina powder or fine sandpaper, rinse again, dry completely, and store it in a dry, dust-free environment.
The core principle behind graphite electrode maintenance is not just cleaning, but preserving the integrity of its active surface. A disciplined post-experiment protocol prevents contamination and surface degradation, which are the primary causes of inaccurate and non-repeatable results.

The Standard Post-Experiment Cleaning Protocol
A consistent cleaning routine is fundamental to the longevity and performance of your electrode. These steps should be performed immediately after the experiment concludes.
Step 1: Immediate Rinsing
The first and most critical step is to rinse the electrode surface with deionized or ultrapure water.
This action removes the bulk of the electrolyte and any loosely adhered reactants before they have a chance to dry or adsorb permanently onto the graphite surface.
Step 2: Addressing Stubborn Contaminants
For residues that water cannot remove, a solvent wash is necessary.
Use an appropriate solvent, such as ethanol or acetone, to dissolve organic films or other stubborn contaminants. Apply the solvent, then follow with another thorough rinse with deionized water.
Step 3: Complete Drying
Once cleaned, the electrode must be dried completely.
Gently pat it dry with a clean, lint-free paper towel or use a stream of clean, compressed air. This prevents ambient moisture from interfering with the electrode surface or introducing impurities during storage.
When and How to Restore the Electrode Surface
Over time, chemical cleaning may become insufficient due to surface fouling or passivation. In these cases, a gentle mechanical cleaning is required to expose a fresh, active surface.
The Need for Mechanical Cleaning
If you observe a decline in performance, such as peak shifting or decreased current response, it often indicates the electrode surface is contaminated or has degraded.
Mechanical cleaning physically removes the compromised top layer of the graphite.
Polishing with Alumina
One common method is polishing with a fine polishing powder, such as alumina.
Create a slurry of the powder with deionized water on a polishing pad. Gently rub the electrode in a figure-eight motion with moderate pressure to renew the surface.
Sanding with Fine-Grit Paper
Alternatively, you can gently sand the electrode tip with very fine-grit sandpaper (e.g., 600-grit or finer).
Ensure you hold the electrode perpendicular to the paper to maintain a flat, even surface. This method is effective but more aggressive than polishing.
The Critical Final Rinse
After any polishing or sanding, the electrode is covered in fine abrasive particles.
It is absolutely essential to rinse it extensively with deionized water, perhaps with the aid of sonication, to remove all traces of the polishing material before drying.
Understanding the Trade-offs and Pitfalls
Proper maintenance requires a nuanced approach. Aggressive or improper cleaning can cause more harm than good.
The Risk of Over-Polishing
Graphite is a relatively soft, brittle material. Excessive or forceful sanding and polishing will abrade the electrode, changing its surface area and geometry.
This can alter its electrochemical behavior and make it difficult to achieve reproducible results. Always use minimal force.
Preventing Cross-Contamination
The goal of cleaning is to remove contaminants, not introduce new ones.
Ensure your deionized water, solvents, and polishing materials are pure. Use clean beakers and handle the electrode with clean gloves or forceps to avoid transferring oils and residues.
The Importance of Inspection
Regularly inspect the electrode's surface under good lighting. Look for discoloration, pitting, or adhered films.
This visual check helps you decide whether a simple rinse is sufficient or if a more thorough mechanical cleaning is warranted.
A Cleaning Strategy for Your Needs
The right cleaning protocol depends on the nature of your experiment. Tailor your approach to maintain the electrode in optimal condition for its next use.
- If your primary focus is routine analysis in aqueous solutions: A thorough rinse with deionized water followed by proper drying is typically sufficient after each use.
- If you are working with organic compounds or polymers: Use an appropriate solvent rinse (like ethanol or acetone) after the initial water rinse to prevent film formation.
- If you observe poor performance or visible surface fouling: Perform a gentle mechanical cleaning by polishing or sanding to expose a fresh, active surface.
Proper electrode care is the foundation of reliable and repeatable electrochemical data.
Summary Table:
| Cleaning Step | Purpose | Key Details |
|---|---|---|
| Immediate Rinsing | Remove bulk contaminants | Use deionized or ultrapure water immediately after use. |
| Solvent Wash | Dissolve stubborn residues | Use ethanol or acetone for organic films, followed by water rinse. |
| Mechanical Polishing | Restore active surface | Use alumina slurry or fine-grit sandpaper for fouled surfaces. |
| Final Rinse & Dry | Prepare for storage | Rinse thoroughly to remove abrasives; dry completely with lint-free cloth or air. |
| Proper Storage | Prevent degradation | Store in a dry, dust-free environment. |
Achieve Peak Performance and Longevity for Your Lab Equipment
Proper maintenance is key to reliable data. KINTEK specializes in high-quality lab equipment and consumables, including the precise tools needed for electrode care. Our products are designed to support the rigorous demands of your laboratory, ensuring accuracy and repeatability in every experiment.
Let us help you maintain the integrity of your research.
Contact our experts today to find the right solutions for your laboratory's specific needs.
Visual Guide
Related Products
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Gold Disc Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- Metal Disc Electrode Electrochemical Electrode
People Also Ask
- What are the characteristics and applications of a graphite sheet electrode? Maximize Reaction Area for Bulk Electrolysis
- What are the properties and applications of a graphite disk electrode? Precision Tools for Electroanalysis
- What are the features and common uses of a graphite rod electrode? A Guide to Durable, Simple Electrochemistry
- What are the properties of graphite rods? Leverage High Conductivity for Extreme Applications
- What are the potential risks when using a graphite electrode in electrochemical tests? Avoid Decomposition and Contamination



















