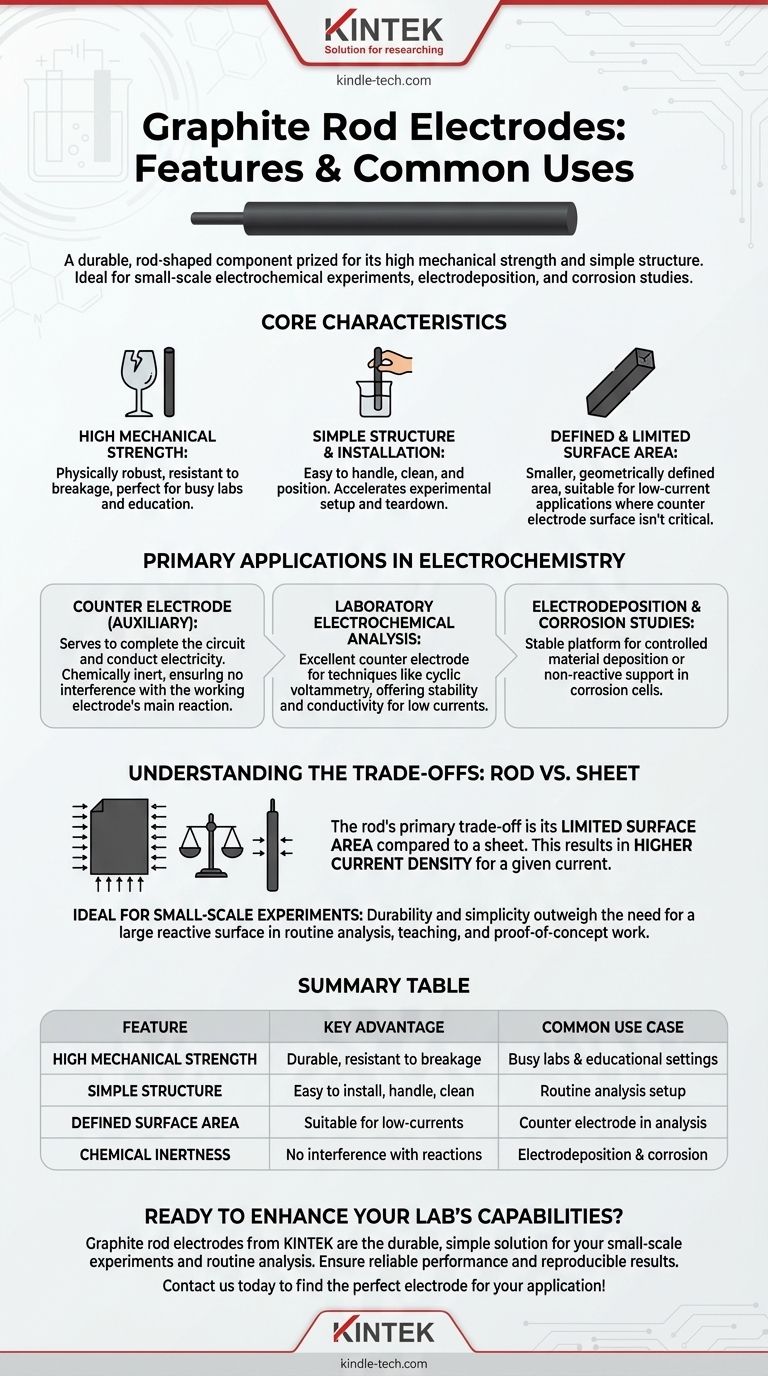
A graphite rod electrode is a durable, rod-shaped component prized for its high mechanical strength and simple structure. It is easy to install and handle, making it a standard choice for a wide range of small-scale laboratory experiments, including electrochemical analysis, electrodeposition, and corrosion studies.
The core decision of whether to use a graphite rod electrode hinges on a trade-off between simplicity and surface area. The rod is the go-to choice for routine, small-scale experiments where durability and ease of use are more critical than a large reactive surface.

Core Characteristics of a Graphite Rod Electrode
A graphite rod's physical properties directly dictate its ideal applications. Understanding these traits is key to using it effectively.
High Mechanical Strength
The solid, rod-shaped structure of this electrode makes it physically robust. It is less prone to breaking than more fragile forms, which is a significant advantage in busy lab environments or educational settings.
Simple Structure and Installation
Its straightforward design makes it exceptionally easy to handle, clean, and position within an electrochemical cell. This simplicity accelerates experimental setup and teardown, improving efficiency for routine analysis.
Defined and Limited Surface Area
Unlike a sheet, a rod has a smaller and more geometrically defined surface area. This is suitable for experiments where the counter electrode's role is simply to complete the circuit without requiring a massive area for reaction.
Primary Applications in Electrochemistry
The graphite rod is a versatile tool, but it excels in specific roles, most commonly as an inert support electrode.
The Role as a Counter Electrode
In a typical three-electrode electrochemical setup, the graphite rod serves as the counter electrode (or auxiliary electrode). Its purpose is to conduct electricity and complete the circuit, allowing the primary reaction of interest to occur at the working electrode. Its chemical inertness ensures it does not interfere with this main reaction.
Laboratory Electrochemical Analysis
For techniques like cyclic voltammetry or other analytical methods, the focus is on the working electrode. A graphite rod is an excellent counter electrode in these cases because it is stable, conductive, and provides the necessary surface area for the low currents typically involved.
Electrodeposition and Corrosion Studies
These applications often benefit from the rod's simple, durable form. It provides a stable platform for controlled deposition of materials or for serving as a non-reactive component in a cell designed to study the corrosion of another material.
Understanding the Trade-offs: Rod vs. Sheet
The most critical decision is choosing the right electrode geometry for your goal. The primary trade-off for a graphite rod is its limited surface area.
Surface Area Limitations
The key disadvantage of a rod is its relatively small surface area compared to a graphite sheet. This makes it unsuitable for applications that demand high currents or a large reaction volume, such as bulk electrolysis or large-scale electrosynthesis.
Current Density Impact
For a given electrical current, the smaller surface area of a rod results in a higher current density (current per unit of area). In some analytical contexts this is acceptable, but for large-scale synthesis, it can be a significant limiting factor that a high-surface-area sheet electrode would solve.
Ideal for Small-Scale Experiments
The rod's characteristics make it the perfect choice for proof-of-concept experiments, educational demonstrations, and routine analytical work. In these scenarios, its durability and simplicity outweigh the need for a large reactive surface.
Making the Right Choice for Your Goal
Select your electrode based on the specific demands of your electrochemical experiment.
- If your primary focus is routine analysis, teaching, or small-scale deposition: The graphite rod is the ideal choice due to its durability, low cost, and ease of use.
- If your primary focus is large-scale electrosynthesis or catalysis: The rod's limited surface area is a major drawback; a graphite sheet electrode is the more appropriate tool.
- If you are building a standard three-electrode cell for analysis: A graphite rod is a reliable and common choice for the counter electrode, where its role is to support the main reaction without interference.
Choosing the correct electrode geometry is fundamental to achieving reliable and reproducible electrochemical results.
Summary Table:
| Feature | Key Advantage | Common Use Case |
|---|---|---|
| High Mechanical Strength | Durable and resistant to breakage | Ideal for busy labs and educational settings |
| Simple Structure | Easy to install, handle, and clean | Accelerates setup for routine analysis |
| Defined Surface Area | Suitable for low-current applications | Perfect as a counter electrode in analytical techniques |
| Chemical Inertness | Does not interfere with main reactions | Essential for electrodeposition and corrosion studies |
Ready to enhance your lab's electrochemical capabilities?
Graphite rod electrodes from KINTEK are the durable, simple solution for your small-scale experiments, educational demos, and routine analysis. Whether you're conducting electrochemical analysis, electrodeposition, or corrosion studies, our high-quality lab equipment ensures reliable performance and reproducible results.
Contact us today to find the perfect electrode for your specific application and discover how KINTEK can support your laboratory's needs with precision and expertise!
Visual Guide
Related Products
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Gold Disc Electrode
- Glassy Carbon Electrochemical Electrode
People Also Ask
- What technical advantages do carbon graphite electrodes offer for electroactive biofilms? Optimize Your Bio-Research
- Why is a high-purity graphite rod preferred as a counter electrode? Ensure Uncontaminated Electrochemical Analysis
- What are the properties of graphite rods? Leverage High Conductivity for Extreme Applications
- What is the primary function of high-purity graphite electrodes in AC leaching? Powering Efficient Metal Recovery
- What are the potential risks when using a graphite electrode in electrochemical tests? Avoid Decomposition and Contamination



















