In most electrochemical systems, a graphite electrode's typical role is to serve as the counter electrode (CE), also known as the auxiliary electrode. Its primary function is to complete the electrical circuit by passing current to or from the working electrode, allowing the reaction of interest to proceed without interfering with the potential measurement.
The counter electrode is a critical but often overlooked component. Its purpose is not to be studied, but to act as a support system, providing or accepting electrons to balance the reaction at the working electrode while ensuring the reference electrode remains stable.

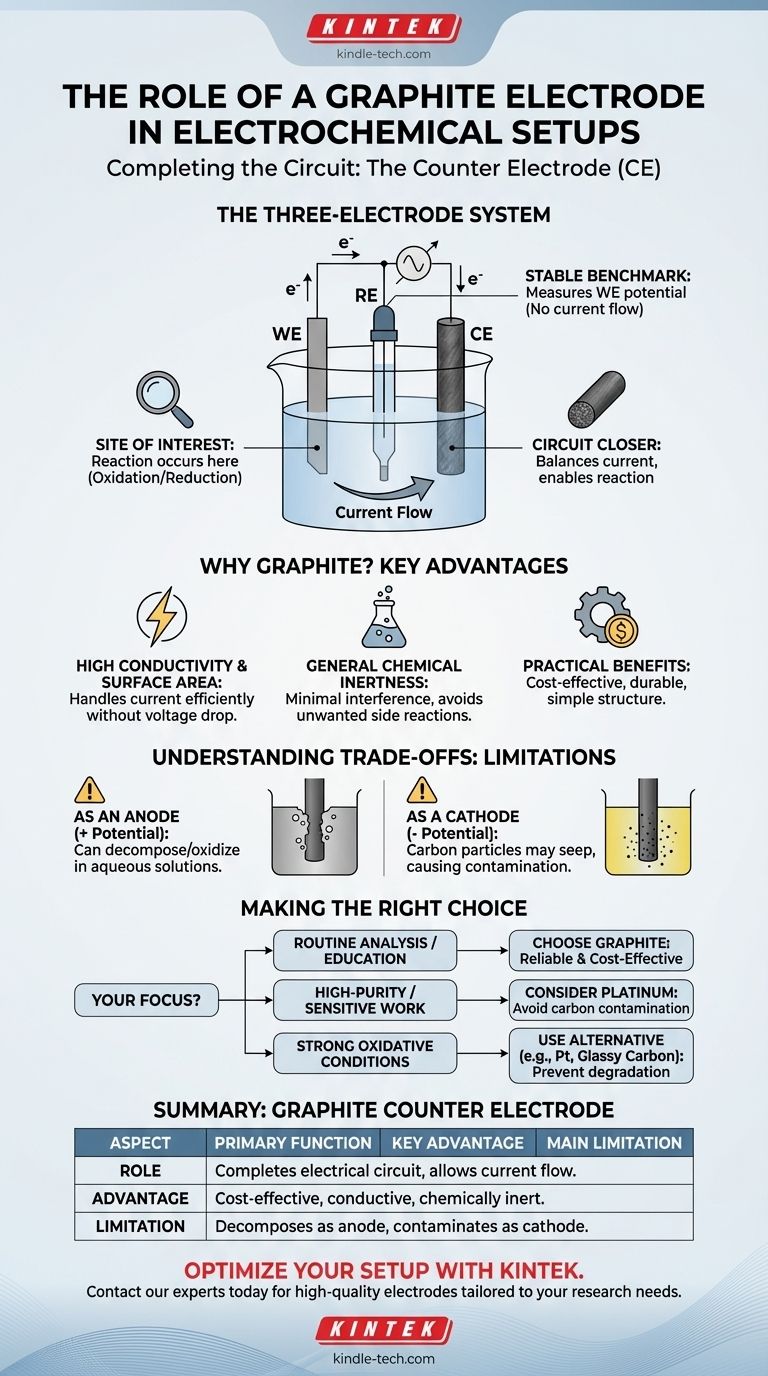
The Three-Electrode System: A Framework for Control
To understand the role of the graphite counter electrode, we must first understand the function of all three electrodes in a standard electrochemical cell. This setup is designed to precisely control and measure the electrochemical events at one specific surface.
The Working Electrode (WE): The Site of Interest
The working electrode is the centerpiece of your experiment. It is the electrode where the specific electrochemical reaction you are studying—such as oxidation or reduction—takes place.
This is the material of interest, often a metal like platinum or a specific alloy, whose properties you are investigating.
The Reference Electrode (RE): The Stable Benchmark
The reference electrode provides a stable, constant potential against which the potential of the working electrode is measured and controlled.
Crucially, a device called a potentiostat ensures that virtually no current flows through the reference electrode. This isolation is essential for maintaining its stable potential throughout the experiment.
The Counter Electrode (CE): The Circuit Closer
The counter electrode's job is to balance the current flowing at the working electrode. If the working electrode is undergoing reduction (gaining electrons), the counter electrode will undergo oxidation (losing electrons), and vice-versa.
By serving as the electron source or sink, the graphite counter electrode completes the electrical circuit. This allows significant current to flow between the working and counter electrodes without involving the delicate reference electrode.
Why Is Graphite a Common Choice for the Counter Electrode?
Graphite is frequently chosen for this role due to a combination of practical and electrochemical properties that make it well-suited for a supporting role.
High Conductivity and Surface Area
Graphite is an excellent electrical conductor and can be formed into shapes with a large surface area. This allows it to handle the required current for the experiment without a significant voltage drop, ensuring the system remains efficient.
General Chemical Inertness
Ideally, a counter electrode should not react with the solvent or electrolyte. Graphite is relatively inert under many conditions, so it can perform its function of passing current without introducing unwanted side reactions that could contaminate the experiment.
Practical Advantages: Cost and Durability
Graphite rod electrodes are known for their simple structure, high mechanical strength, and ease of use. Compared to alternatives like platinum, graphite is significantly more cost-effective, making it ideal for routine lab work, educational settings, and small-scale experiments.
Understanding the Trade-offs: When Graphite Isn't Ideal
While highly useful, graphite is not a perfect material. Its limitations are critical to understand for ensuring experimental accuracy.
Decomposition as an Anode
If the graphite electrode is held at a positive potential (acting as the anode), it can be oxidized and decompose, especially in aqueous solutions where water and oxygen are present. This degradation can interfere with your results.
Contamination as a Cathode
When used as the cathode (negative potential), the electrode itself is not damaged. However, small particles of the carbon material may seep into the solution, sometimes causing it to turn yellow. This can be a major issue in high-purity applications like electrodeposition or trace analysis.
When to Choose an Alternative
For high-precision experiments where any contamination is unacceptable or where strong oxidizing conditions are present, a more inert material like a platinum (Pt) wire or mesh is often used as the counter electrode, despite its significantly higher cost.
Making the Right Choice for Your Experiment
Selecting the right counter electrode requires balancing cost, performance, and the specific demands of your electrochemical test.
- If your primary focus is routine analysis or educational labs: Graphite is an excellent, cost-effective choice for the counter electrode due to its reliability and low cost.
- If your primary focus is high-purity electrodeposition or sensitive analysis: Consider a platinum counter electrode to avoid potential carbon contamination from a graphite cathode.
- If your primary focus involves strong oxidative potentials: Be cautious with graphite as an anode, as it can degrade; a platinum or glassy carbon electrode might be a more stable choice.
Understanding the specific role and limitations of each component empowers you to design a more robust and reliable electrochemical experiment.
Summary Table:
| Aspect | Role of Graphite Counter Electrode |
|---|---|
| Primary Function | Completes the electrical circuit, allowing current to flow to/from the working electrode. |
| Key Advantage | Cost-effective, high conductivity, and chemically inert under many conditions. |
| Main Limitation | Can decompose as an anode or contaminate solution as a cathode in sensitive applications. |
| Ideal Use Case | Routine lab analysis, educational settings, and small-scale experiments. |
Optimize your electrochemical setup with the right components.
Understanding the precise role of each electrode is key to obtaining reliable data. KINTEK specializes in providing high-quality lab equipment and consumables, including a range of electrodes tailored for various electrochemical applications.
Whether you need cost-effective graphite electrodes for routine analysis or high-purity platinum alternatives for sensitive work, we have the expertise and products to support your research. Let us help you design a more robust and accurate experiment.
Contact our experts today to discuss your specific needs and ensure your lab is equipped for success.
Visual Guide
Related Products
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Glassy Carbon Electrochemical Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- What are the properties of graphite rods? Leverage High Conductivity for Extreme Applications
- What are the properties and applications of a graphite disk electrode? Precision Tools for Electroanalysis
- What technical advantages do carbon graphite electrodes offer for electroactive biofilms? Optimize Your Bio-Research
- What are the features and common uses of a graphite rod electrode? A Guide to Durable, Simple Electrochemistry
- What are the potential risks when using a graphite electrode in electrochemical tests? Avoid Decomposition and Contamination



















