In the Chemical Vapor Deposition (CVD) process, a precursor is the essential chemical ingredient that contains the atoms you want to deposit. It is a volatile compound—which can be a gas, liquid, or solid—that is transported in a vapor phase into a reaction chamber. Once inside, it decomposes on a heated surface (the substrate), leaving behind a solid thin film of the desired material while the remaining chemical components are removed as waste gas.
A precursor is best understood as the critical delivery vehicle in thin-film manufacturing. Its specific chemical makeup not only determines what material is deposited but also dictates the purity, structure, and quality of the final layer, making its selection the most fundamental choice in any CVD process.

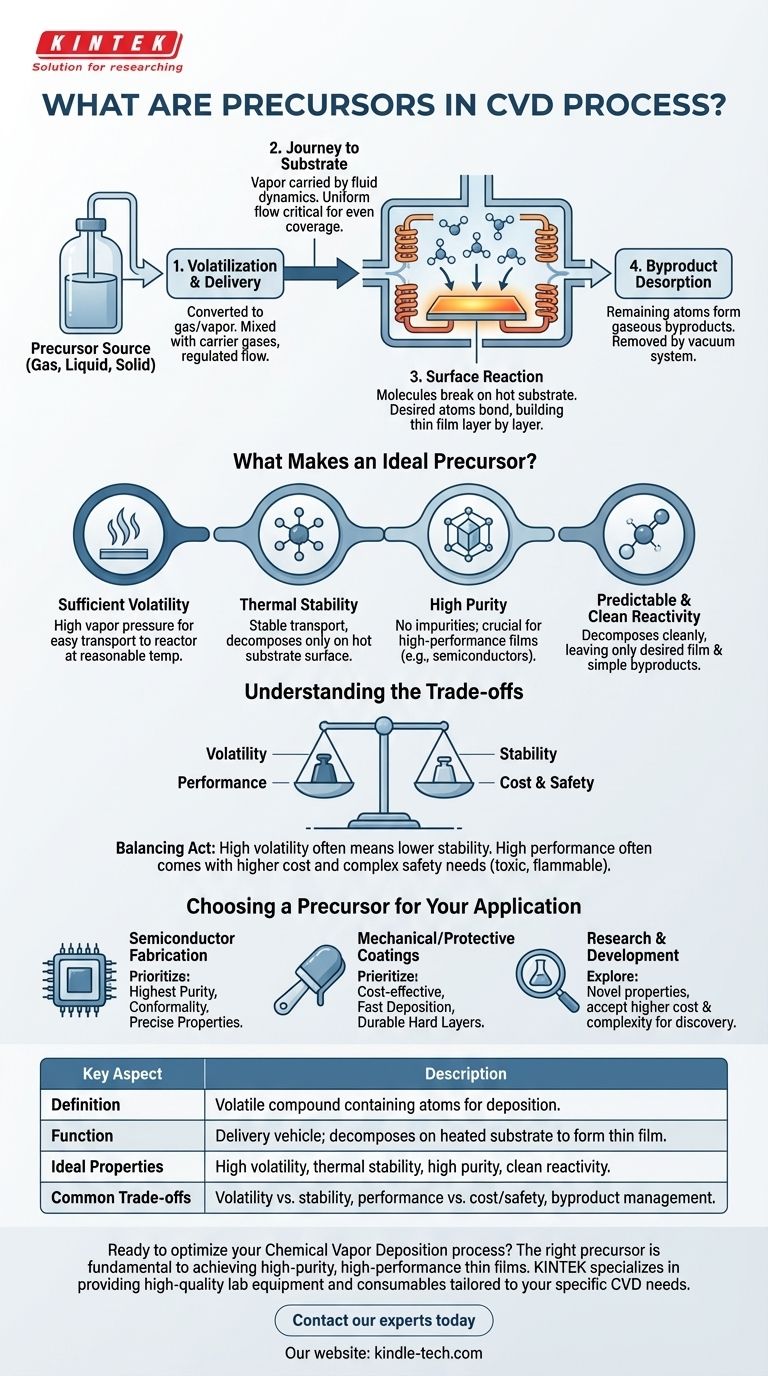
The Role of the Precursor in the CVD Workflow
To understand what a precursor does, it's helpful to follow its journey through the four key stages of the CVD process.
The Starting Point: Volatilization and Delivery
The process begins by converting the precursor into a gas. Whether it starts as a liquid, solid, or gas, it must be volatile enough to be controllably transported into the CVD reactor.
This vapor is then fed into the reactor chamber, often mixed with carrier gases that help regulate its flow and concentration.
The Journey to the Substrate
Inside the reactor, fluid dynamics carry the precursor molecules toward the target substrate.
This step is critical for ensuring uniform coverage. The gas must flow evenly over the entire surface to avoid variations in the thickness of the final film.
The Critical Moment: Surface Reaction
When the precursor molecules come into contact with the heated substrate, they gain enough energy to trigger a chemical reaction.
This reaction breaks the chemical bonds within the precursor, causing the desired atoms to "stick" and bond to the surface. This atomic-level deposition is how the thin film is built, layer by layer.
The Cleanup: Byproduct Desorption
The precursor molecule is designed to leave behind only a specific element. All other atoms from the original molecule form gaseous byproducts.
These byproducts must be effectively removed from the chamber by a vacuum system. If they linger, they can contaminate the film or interfere with the ongoing deposition process.
What Makes an Ideal Precursor?
The success of a CVD process hinges entirely on the properties of the precursor. Engineers and chemists look for a specific combination of characteristics.
Sufficient Volatility
The precursor must have a high enough vapor pressure to be easily transported into the reactor at a reasonable temperature. If it's not volatile, it simply can't be delivered to the substrate efficiently.
Thermal Stability
There is a crucial balance here. The precursor must be stable enough to travel through the gas lines to the reactor without decomposing prematurely.
The decomposition should happen only on the hot substrate surface, not before. This ensures the deposition is localized and controlled.
High Purity
Any impurity within the precursor material will almost certainly be incorporated into the final film, degrading its performance.
For applications like semiconductors, where even parts-per-billion contamination can cause device failure, precursor purity is non-negotiable.
Predictable and Clean Reactivity
An ideal precursor decomposes cleanly, leaving behind the desired film and simple, non-reactive gaseous byproducts.
Complex or unwanted side reactions can introduce impurities, damage the substrate, or create hazardous waste products that are difficult to handle.
Understanding the Trade-offs
Selecting a precursor is rarely straightforward, as ideal properties often conflict with practical realities.
Volatility vs. Stability
The most common trade-off is between volatility and stability. Often, compounds that are highly volatile (easy to turn into a gas) are also less thermally stable, making them prone to decomposing before they reach the substrate.
Finding a molecule in the "sweet spot" is a central challenge in precursor design.
Performance vs. Cost and Safety
The highest-performing precursors are frequently expensive to synthesize. Furthermore, many are toxic, flammable, or even pyrophoric (igniting on contact with air).
This necessitates complex and costly safety equipment and handling protocols, which adds significantly to the overall cost of manufacturing.
Byproduct Management
The "waste" products of the precursor reaction are a major consideration. Corrosive byproducts like hydrochloric acid (HCl) can damage the equipment over time.
Toxic or environmentally harmful gases require expensive abatement systems to treat the exhaust before it can be released, adding another layer of complexity to the process.
Choosing a Precursor for Your Application
The right choice is always dictated by the end goal. Your specific application determines which precursor properties you must prioritize.
- If your primary focus is semiconductor fabrication: You must prioritize precursors that offer the highest possible purity and result in highly conformal films with precise electrical properties.
- If your primary focus is mechanical or protective coatings: You may prioritize precursors that are cost-effective, deposit material quickly, and create hard, durable layers, even if absolute purity is less critical.
- If your primary focus is research and development: You might explore novel or custom-synthesized precursors to achieve new material properties, accepting higher costs and handling complexities as part of the discovery process.
Ultimately, mastering the CVD process begins with a deep understanding of the precursor, the foundational element that dictates the final outcome.
Summary Table:
| Key Aspect | Description |
|---|---|
| Definition | A volatile chemical compound containing the atoms to be deposited. |
| Function | Acts as a delivery vehicle, decomposing on a heated substrate to form a thin film. |
| Ideal Properties | High volatility, thermal stability, high purity, and clean reactivity. |
| Common Trade-offs | Volatility vs. stability, performance vs. cost/safety, and byproduct management. |
Ready to optimize your Chemical Vapor Deposition process?
The right precursor is fundamental to achieving high-purity, high-performance thin films for semiconductors, protective coatings, and advanced materials research. KINTEK specializes in providing high-quality lab equipment and consumables tailored to your specific CVD needs.
Contact our experts today to discuss how we can support your laboratory's success with reliable solutions and expert guidance.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- CVD Diamond Domes for Industrial and Scientific Applications
People Also Ask
- What is plasma activated chemical vapor deposition? Enable Low-Temperature Thin Film Deposition
- How does RF power create plasma? Achieve Stable, High-Density Plasma for Your Applications
- What is the plasma CVD process? Achieve Low-Temperature Thin Film Deposition
- Why does PECVD commonly use RF power input? For Precise Low-Temperature Thin Film Deposition
- What are the advantages of plasma enhanced CVD? Enable Low-Temperature, High-Quality Thin Film Deposition



















