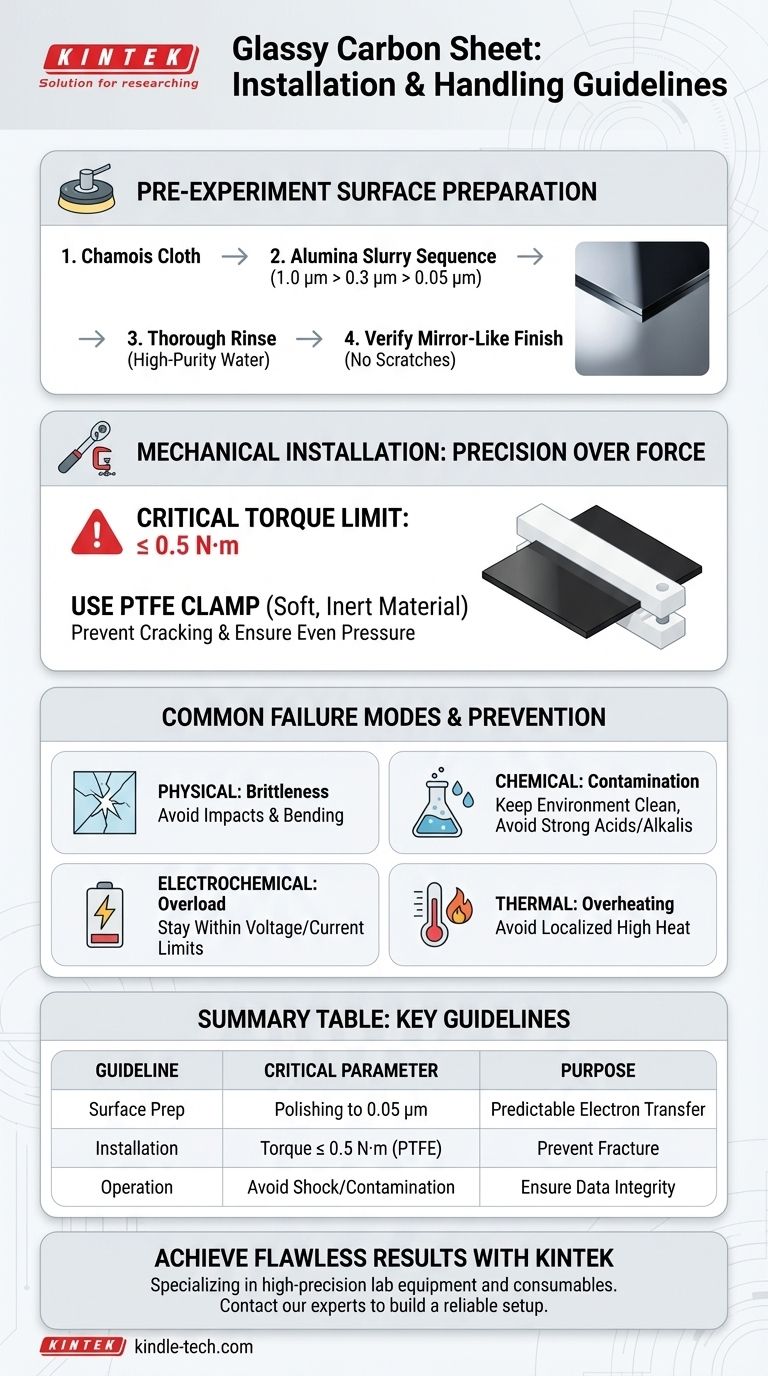
Properly installing and handling a glassy carbon sheet is a matter of precision and control. To prevent fracture, you must install it using a PTFE clamp with an applied torque no greater than 0.5 N·m. Before use, the sheet requires sequential polishing to a mirror finish, and during operation, you must avoid physical shock, chemical contamination, and excessive electrical load to ensure data integrity.
Glassy carbon's value lies in its inert, conductive surface, but its brittleness and sensitivity to fouling are its greatest liabilities. Success depends not on a single step, but on a holistic protocol that protects its physical and chemical integrity from preparation through analysis.

The Foundation: Pre-Experiment Surface Preparation
The state of the electrode surface directly dictates the quality and reproducibility of your electrochemical measurements. An unpolished or contaminated surface will yield unreliable data.
Why Polishing is Non-Negotiable
A pristine, smooth surface is essential for achieving predictable electron transfer kinetics. Any microscopic scratches, embedded polishing media, or adsorbed contaminants from previous experiments will create active sites that interfere with your results.
The Correct Polishing Sequence
Begin polishing on a chamois cloth. Work through a sequence of alumina (Al₂O₃) suspensions, moving from coarse to fine to achieve a flawless finish.
A standard, effective sequence is 1.0 µm, followed by 0.3 µm, and finishing with 0.05 µm (50 nm) slurry. After the final polishing step, rinse the sheet thoroughly with high-purity water to remove all particulate matter.
Verifying the Surface
A properly prepared glassy carbon sheet will have a perfectly reflective, mirror-like finish. Tilt the sheet under a light source; you should see no visible scratches, haziness, or smudges. This visual check is your first confirmation of a well-prepared electrode.
Mechanical Installation: Precision Over Force
The primary cause of glassy carbon sheet failure is physical damage during installation. Its glass-like, brittle nature means it cannot tolerate uneven pressure or excessive force.
The Critical Torque Limit
Always use a torque wrench when securing the clamp. The maximum applied torque should never exceed 0.5 N·m. Overtightening is the most common cause of catastrophic cracking and failure.
Choosing the Right Clamp
Use a clamp made of a soft, inert material like PTFE (polytetrafluoroethylene). This material distributes the clamping force more evenly and will not react with your electrolyte or contaminate the electrode surface.
Controlling the Exposed Area
For quantitative electrochemical analysis, the electrochemically active surface area must be known precisely. Ensure your setup controls this exposed area with an error of less than 3%. Inconsistent area exposure between experiments is a major source of error in calculating current density.
Understanding the Trade-offs: Common Failure Modes
To ensure longevity and reliable data, you must be aware of the material's inherent weaknesses and operate within its limits.
Physical Failure: Brittleness and Fracture
Glassy carbon has virtually no ductility. It will fracture without warning if subjected to sharp impacts, excessive bending, or torsion. Handle it as you would a delicate piece of glassware.
Chemical Failure: Contamination and Fouling
The electrode surface is highly susceptible to fouling from organic substances and metal compounds. Maintain a clean experimental environment and use high-purity solutions. Avoid immersing the sheet in strong acid or alkali solutions for prolonged periods, as this can slowly degrade the surface.
Electrochemical Failure: Exceeding Operational Limits
Every electrode/solvent system has a stable potential window. Operating outside the specified current and voltage limits can cause irreversible reactions on the electrode surface, solvent breakdown, or material damage, permanently altering its electrochemical behavior.
Thermal Failure: Overheating
Glassy carbon is stable at high temperatures in inert environments but can be damaged by localized, direct contact with high-temperature sources. This can induce thermal stress and cause fractures or degradation.
Making the Right Choice for Your Goal
Your specific experimental goal will determine which handling aspects require the most attention.
- If your primary focus is analytical accuracy: Meticulous surface polishing and precise control of the exposed electrode area are your most critical tasks.
- If your primary focus is electrode longevity: Strictly adhere to the 0.5 N·m torque limit and operate well within the material's chemical, thermal, and electrical limits.
- If you are troubleshooting inconsistent results: Re-examine your polishing and cleaning protocol first, as surface fouling is the most common culprit for poor reproducibility.
By treating the glassy carbon sheet not as a simple component but as a precision instrument, you ensure the integrity of your data and the longevity of your investment.
Summary Table:
| Key Handling Guideline | Critical Parameter | Purpose |
|---|---|---|
| Surface Preparation | Sequential polishing to 0.05 µm | Achieve a mirror finish for predictable electron transfer |
| Mechanical Installation | Torque ≤ 0.5 N·m with PTFE clamp | Prevent catastrophic cracking and fracture |
| Operational Limits | Avoid physical shock, contamination, and excessive electrical load | Ensure data integrity and electrode longevity |
Achieve flawless electrochemical results with the right lab equipment.
Properly handling sensitive components like glassy carbon sheets is essential for data accuracy. KINTEK specializes in high-precision lab equipment and consumables, including the clamps and polishing materials needed to protect your investment and ensure reproducible results.
Let our experts help you build a reliable experimental setup. Contact KINTEK today to discuss your laboratory's specific needs.
Visual Guide
Related Products
- Glassy Carbon Electrochemical Electrode
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Hydrophilic Carbon Paper TGPH060 for Battery Lab Applications
- Silicon Carbide (SIC) Ceramic Sheet Wear-Resistant Engineering Advanced Fine Ceramics
- Copper Sulfate Reference Electrode for Laboratory Use
People Also Ask
- What are the pre-treatment steps for a glassy carbon electrode before use? Ensure Reliable Electrochemical Data
- What is a glassy carbon electrode made of? The Engineered Material Powering Electrochemical Analysis
- Why is glassy carbon selected for mediator-assisted indirect oxidation of glycerol? The Key to Unbiased Research
- How is a glassy carbon electrode activated before an experiment? Achieve Clean, Reproducible Electrochemical Data
- How to make a glassy carbon electrode? A Guide to the Industrial Pyrolysis Process



















