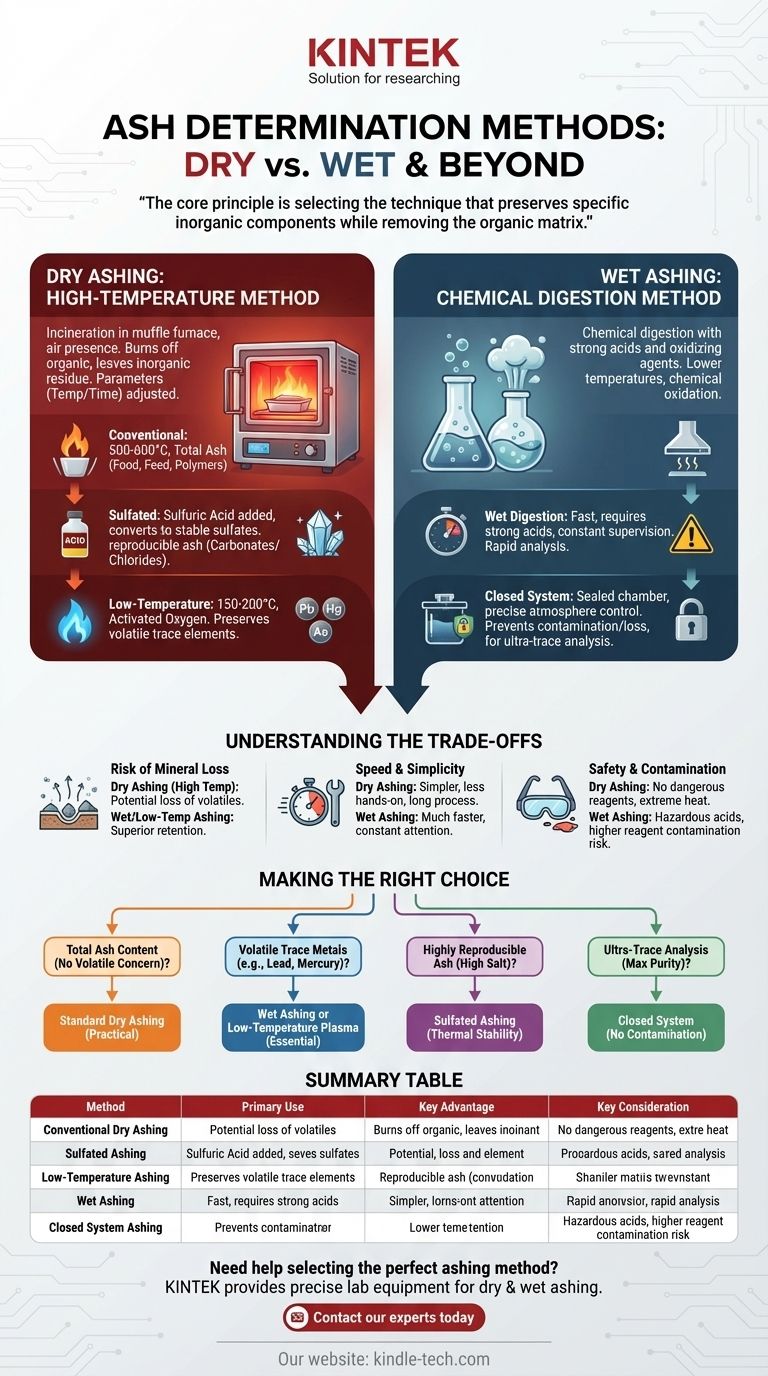
The primary methods for ash determination are broadly categorized into dry ashing and wet ashing. These two approaches encompass several specific techniques, including conventional high-temperature ashing, sulfated ashing, low-temperature ashing, and closed system ashing, with the best choice depending on the sample being analyzed and the goal of the test.
The core principle of ash determination is not finding the single "best" method, but rather selecting the appropriate technique that preserves the specific inorganic components you intend to measure while completely removing the organic matrix.

The Two Core Approaches: Dry vs. Wet Ashing
Fundamentally, all ashing techniques are designed to eliminate the organic matter from a sample, leaving only the inorganic mineral residue, or ash. The two main strategies to achieve this use either extreme heat or chemical digestion.
Dry Ashing: The High-Temperature Method
Dry ashing is the most common method. It uses a high-temperature muffle furnace to incinerate the organic components of the sample in the presence of air.
This process essentially burns off everything but the inorganic minerals. The parameters, such as furnace temperature and time, are adjusted based on the specific sample type.
Wet Ashing: The Chemical Digestion Method
Wet ashing, also known as wet digestion, uses strong acids and oxidizing agents to break down the organic matrix of a sample.
This technique is performed at much lower temperatures than dry ashing. It is a chemical oxidation process rather than a thermal one.
Exploring Specific Ashing Techniques
While most methods fall under the dry or wet ashing umbrella, several distinct techniques are used for specific analytical purposes.
Conventional Dry Ashing
This is the standard high-temperature procedure, typically conducted at temperatures between 500°C and 600°C. It is widely used for determining the total mineral content in materials like food, feed, and polymers.
Sulfated Ashing
This is a specialized form of dry ashing where sulfuric acid is added to the sample before heating. The acid converts metal salts to sulfates, which are more thermally stable.
This technique is often used to obtain a more reproducible ash residue, especially for samples containing carbonates or chlorides which can be volatile at high temperatures.
Low-Temperature Ashing
To avoid the loss of volatile minerals, low-temperature ashing is performed around 150°C to 200°C. It uses activated oxygen to slowly oxidize the organic matter.
This method is crucial when the analysis is focused on measuring trace elements that would vaporize and be lost at the high temperatures of a conventional muffle furnace.
Closed System Ashing
This technique involves heating the sample in a sealed, airtight chamber. This provides precise control over the atmosphere during incineration.
A closed system is vital for preventing the loss of volatile elements and protecting the sample from atmospheric contamination, making it ideal for ultra-trace mineral analysis.
Understanding the Trade-offs
Choosing the correct method requires understanding the advantages and disadvantages inherent to each approach.
Risk of Mineral Loss
The primary drawback of high-temperature dry ashing is the potential loss of volatile minerals like arsenic, lead, and mercury. Wet ashing or low-temperature ashing is superior for retaining these elements.
Speed and Simplicity
Dry ashing is generally simpler to perform and requires less hands-on attention, allowing for multiple samples to be processed simultaneously. However, the process itself can take many hours.
Wet ashing is typically much faster in terms of digestion time but demands constant operator supervision and involves the hazardous handling of corrosive acids.
Safety and Contamination
Dry ashing involves extreme heat but avoids the use of dangerous chemical reagents. Wet ashing, conversely, requires a fume hood and careful protocols for handling strong, corrosive acids.
Wet ashing also carries a higher risk of reagent-based contamination, where impurities in the acids can be introduced into the sample and affect the final measurement.
Making the Right Choice for Your Analysis
Your analytical goal and sample composition are the only factors that should guide your choice of ashing method.
- If your primary focus is total ash content without concern for volatile elements: Standard dry ashing is the most practical and straightforward choice.
- If your primary focus is analyzing for volatile trace metals like lead or mercury: Wet ashing or low-temperature plasma ashing is essential to prevent their loss.
- If your primary focus is obtaining a highly reproducible ash from a sample with high salt content: Sulfated ashing provides the thermal stability needed for an accurate result.
- If your primary focus is ultra-trace analysis requiring maximum purity: A closed system is necessary to prevent any risk of atmospheric contamination.
Ultimately, the right method is the one that reliably prepares your sample for the specific elemental analysis you need to perform.
Summary Table:
| Method | Primary Use | Key Advantage | Key Consideration |
|---|---|---|---|
| Conventional Dry Ashing | Total ash content in food, feed, polymers | Simple, high-throughput | Potential loss of volatile minerals |
| Sulfated Ashing | Reproducible ash from high-salt samples | Thermally stable sulfate residue | Requires sulfuric acid addition |
| Low-Temperature Ashing | Trace element analysis (e.g., lead, mercury) | Preserves volatile minerals | Slower oxidation process |
| Wet Ashing | Fast digestion for trace metal analysis | Rapid, lower temperature | Requires hazardous acid handling |
| Closed System Ashing | Ultra-trace analysis requiring maximum purity | Prevents contamination and loss | More complex equipment needed |
Need help selecting the perfect ashing method for your lab's analysis?
The right sample preparation is critical for accurate results. KINTEK specializes in providing the precise lab equipment—from robust muffle furnaces for dry ashing to safe digestion systems for wet ashing—that your laboratory needs for reliable ash determination.
Contact our experts today to discuss your application and ensure you have the optimal solution for your specific samples and analytical goals.
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the classification of refractory materials? A Guide to Chemical and Thermal Selection
- What is a muffle furnace used for? Achieve High-Temperature Processing with Purity
- What is furnace lining? The Engineered System Protecting Your High-Temperature Processes
- Why ceramics can withstand high temperature? Unlock the Secrets of Atomic Structure
- Why the melting temperature of ceramic is higher than for most metals? Unpacking Atomic Bond Strength



















