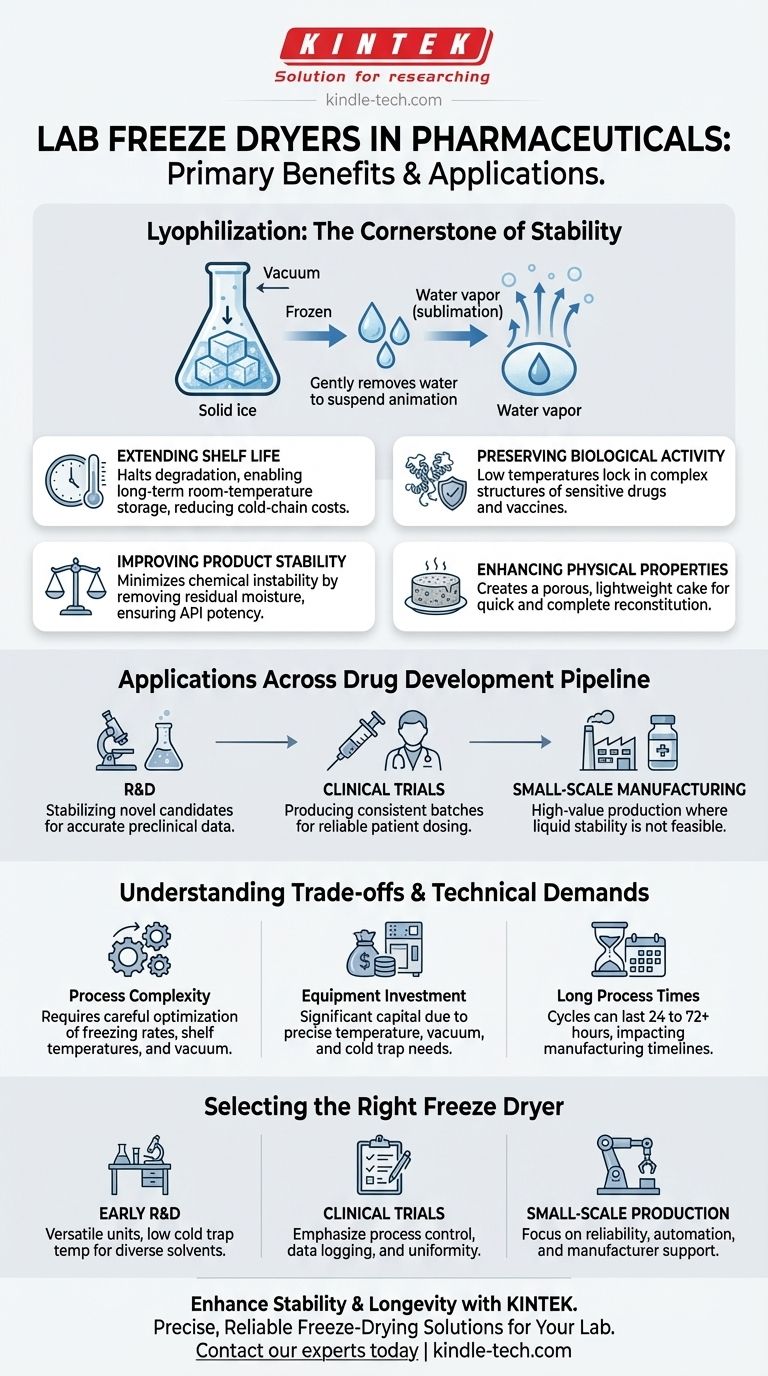
The primary benefits of a lab freeze dryer in pharmaceuticals are extending shelf life, preserving the biological activity of sensitive drugs, improving product stability, and enhancing physical properties for transport and storage. This process, known as lyophilization, is essential for creating therapeutic products that remain potent and safe for years without requiring constant refrigeration.
The fundamental value of freeze-drying is its ability to gently remove water from sensitive biological and chemical compounds. This process puts the product into a state of suspended animation, making it stable for years and preserving its therapeutic integrity until it's reconstituted for use.

Why Freeze-Drying is a Cornerstone of Pharmaceutical Stability
Lyophilization is more than just a drying method; it's a precise stabilization technique. It works by freezing the material and then reducing the surrounding pressure to allow the frozen water to sublimate directly from a solid to a gas, bypassing the damaging liquid phase.
Extending Shelf Life Beyond Refrigeration
By removing water—the primary medium for chemical reactions and microbial growth—freeze-drying effectively halts degradation. This allows complex drugs to be stored at room temperature for extended periods, simplifying logistics and reducing the costs associated with cold-chain storage.
Preserving Delicate Biological Structures
Many modern drugs, especially vaccines and protein-based therapies, are incredibly fragile. High temperatures or the physical stress of conventional evaporation can destroy their complex three-dimensional structures, rendering them useless. Lyophilization is a low-temperature process that locks these structures in place.
Achieving Superior Product Stability
The final freeze-dried product has an extremely low residual moisture content. This minimizes chemical instability, such as hydrolysis, ensuring that the active pharmaceutical ingredient (API) maintains its specified potency throughout its shelf life.
Creating a Consistent and Reusable Product
The process results in a porous, solid "cake" that is lightweight and occupies the same volume as the original frozen liquid. This structure allows the product to be redissolved (reconstituted) quickly and completely, which is critical for clinical applications where accurate dosing is paramount.
Applications Across the Drug Development Pipeline
Lab freeze dryers are not just for large-scale manufacturing; they are a critical tool at every stage of bringing a drug to market.
Research and Development (R&D)
In the early stages, scientists use freeze dryers to stabilize novel drug candidates. This allows them to study the compounds over time without the variable of degradation, ensuring the accuracy of preclinical data.
Clinical Trials
For clinical trials, it is essential to produce consistent and stable batches of investigational drugs. Freeze-drying ensures that every patient in a trial receives the drug at its intended potency, regardless of when or where it is administered.
Small-Scale Manufacturing
Lab-scale freeze dryers are used to produce small commercial batches of high-value products. This includes many vaccines, injectable drugs, and therapies where stability in a liquid form is not feasible.
Understanding the Trade-offs and Technical Demands
While the benefits are significant, adopting lyophilization requires a clear understanding of its complexities.
Process Complexity
Developing a successful freeze-drying cycle is a complex scientific endeavor. It requires careful optimization of freezing rates, shelf temperatures, and vacuum levels for each specific product to avoid collapse or activity loss.
Equipment Investment
Freeze dryers are sophisticated pieces of equipment that represent a significant capital investment. The need for precise temperature control, deep vacuums, and robust cold traps makes them more expensive than simple ovens or evaporators.
Long Process Times
A typical freeze-drying cycle can last from 24 to 72 hours, or even longer. This long processing time can be a bottleneck in production and must be factored into manufacturing timelines.
Selecting the Right Freeze Dryer for Your Application
Choosing the correct equipment depends entirely on your specific goal. Key technical factors include cold trap temperature, vacuum level, and shelf temperature uniformity.
- If your primary focus is early R&D with diverse compounds: Prioritize a versatile unit with a very low cold trap temperature to handle a wide range of solvents and precise controls to develop new cycles.
- If your primary focus is producing materials for clinical trials: Emphasize process control, data logging capabilities, and plate temperature uniformity to ensure batch-to-batch consistency and regulatory compliance.
- If your primary focus is small-scale, quality-controlled production: Look for reliability, automation features to ensure repeatability, and a system from a manufacturer with a strong reputation for service and support.
Ultimately, a lab freeze dryer is an essential tool for transforming unstable liquid formulations into stable, long-lasting products that are safe and effective for patients.
Summary Table:
| Benefit | Key Impact |
|---|---|
| Extends Shelf Life | Enables room-temperature storage, reducing cold-chain costs. |
| Preserves Biological Activity | Protects delicate structures of proteins and vaccines. |
| Improves Product Stability | Minimizes degradation by removing water. |
| Enhances Physical Properties | Creates a porous cake for easy reconstitution and accurate dosing. |
Ready to enhance the stability and longevity of your pharmaceutical formulations? A KINTEK lab freeze dryer is the precise, reliable tool you need for R&D, clinical trials, and small-scale production. We specialize in providing high-quality lab equipment and consumables to meet the exacting demands of the pharmaceutical industry. Contact our experts today to find the perfect freeze-drying solution for your laboratory's unique needs and ensure your therapeutic products remain potent and safe.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- Why is a freeze dryer preferred over thermal drying for Fe-ZTA cermets? Ensure Pure, Homogeneous Slurry Processing
- What are the advantages of using freeze drying for phase change materials with biopolymer shells? Optimize Stability
- Why is a freeze dryer preferred for drying nickel nanoparticle precursors? Prevent Hard Agglomeration Now
- Why is a freeze dryer preferred for reduced graphene oxide (Hh-RGO) powders? Preserve Nano-Structure and Performance
- What role does a laboratory freeze dryer play in the synthesis of graphene-based electrocatalysts? Preserve 3D Structures



















