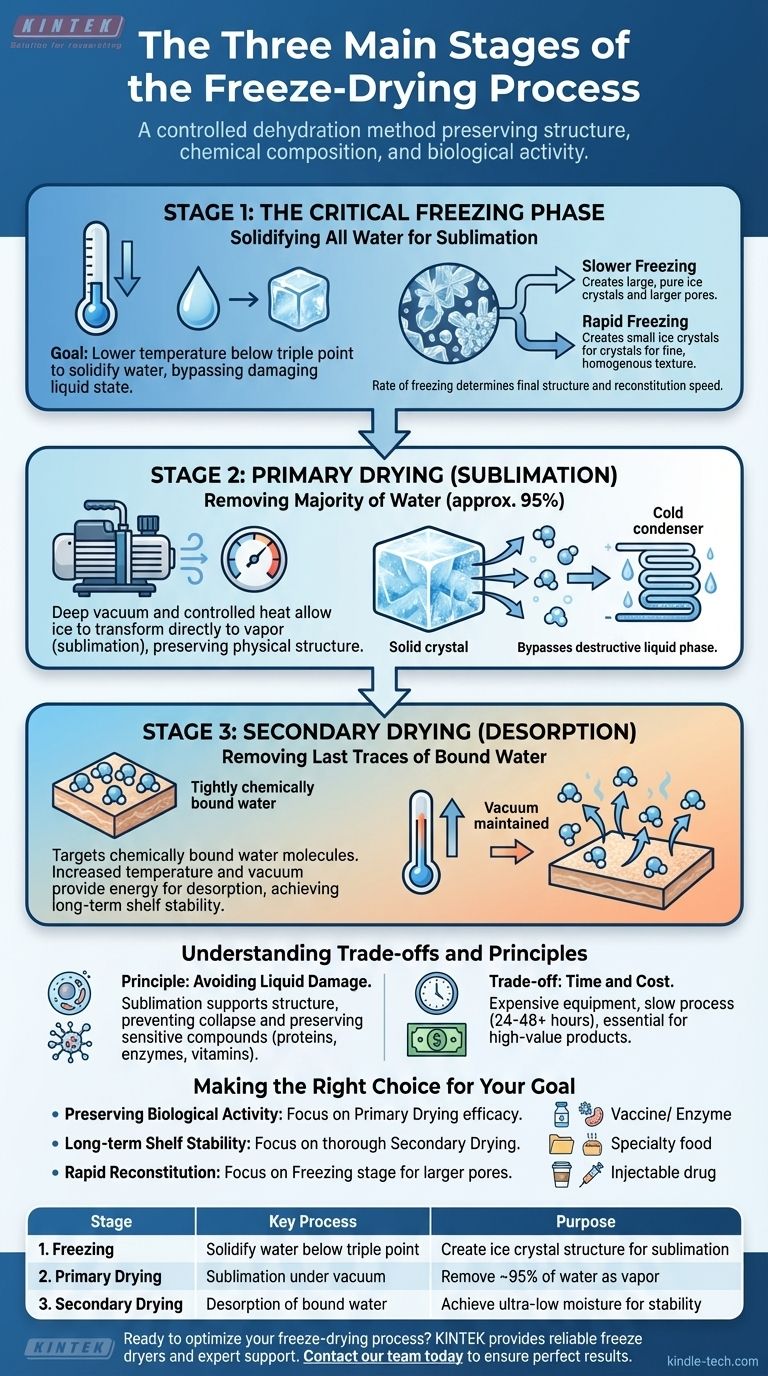
The freeze-drying process is a carefully controlled three-stage method designed to remove water from a substance while preserving its structure, chemical composition, and biological activity. The process is broken down into Freezing, where the material's water is turned市场 into solid ice; Primary Drying, where a vacuum allows that ice to transform directly into vapor (sublimation); and Secondary Drying, where the last traces of bound water molecules are removed (desorption).
Freeze-drying is not simply about removing water; it's a sophisticated dehydration process that bypasses the destructive liquid phase. This preserves a material's delicate structure and biological integrity, making it the superior choice for sensitive applications.

Stage 1: The Critical Freezing Phase
This initial stage sets the foundation for the entire process, and how it is performed directly impacts the quality of the final product.
The Goal: Solidifying All Water
The first and most essential step is to completely freeze the material. The temperature must be lowered below its triple point, the unique temperature and pressure at which a substance can exist simultaneously as a solid, liquid, and gas.
By fully solidifying the water, you prepare it for removal without it ever passing through the damaging liquid state.
The Impact on Final Structure
The rate of freezing determines the size of the ice crystals that form. Slower freezing creates larger, purer ice crystals, which leave behind larger pores after sublimation. Rapid freezing creates smaller ice crystals, resulting in a finer, more homogenous texture.
The size of these pores influences the speed of the subsequent drying stages and how quickly the final product can be reconstituted with water.
Stage 2: Primary Drying (Sublimation)
This is the longest and most energy-intensive stage, where the vast majority of water is removed from the product.
The Role of Vacuum
Once the material is frozen ઉત્પાદન, a deep vacuum is applied to the chamber. This reduces the pressure violência to a level far below water's triple point.
Sublimation in Action
With the pressure held low, a small, controlled amount of heat is introduced. This energy gives the ice molecules just enough energy to transition directly from a solid to a gas, a process called sublimation.
The liquid phase is completely skipped, which is crítico for preserving the product's physical structure. The water vapor is then collected and re-frozen on a condenser coil, removing it from the system.
Removing the Majority of Water
This stage removes approximately 95% of the water from the material. What remains is a porous, lightweight, and structurally intact version of the original substance.
Stage 3: Secondary Drying (Desorption)
This final "polishing" step is crucial for ensuring long-term shelf stability by removing the last remaining water molecules.
Targeting Bound Water
After sublimation, a small amount of water remains, not as ice, but chemically bound to the surface of the material. These molecules could not be removed during primary drying.
How Desorption Works
The temperature is gently raised further, while the vacuum is maintained or even increased. This gives the bound water molecules enough energy to break their bonds with the material and escape as vapor.
This process is known as desorption, and it is essential for achieving the extremely low residual moisture content that defines a properly freeze-dried product.
Understanding the Trade-offs and Principles
The effectiveness of freeze-drying is rooted in one core principle: avoiding the damage caused by liquid water and conventional heat-drying.
Preserving Delicate Structures
Evaporation in normal drying causes surface tension and migration of solutes, which can shrink, crack, and collapse a material's cellular structure. By using sublimation, the solid ice scaffold supports the structure until it is removed, leaving it perfectly intact.
Maintaining Biological Activity
The low-temperature nature of the process is gentle on sensitive compounds. It prevents the degradation of proteins, enzymes, vitamins, and other bioactive molecules that would be destroyed by the heat of conventional drying.
The Cost of Quality
The primary trade-off is time and cost. The equipment is expensive, and the process is slow, often taking 24 to 48 hours or more. This makes it impractical for bulk commodities but essential for high-value products like pharmaceuticals, specialty foods, and biological specimens.
Making the Right Choice for Your Goal
Understanding these stages helps you evaluate if freeze-drying is the right method for your specific material.
- If your primary focus is preserving biological activity (e.g., vaccines, enzymes): The low-temperature sublimation in Primary Drying is the most critical stage for maintaining efficacy.
- If your primary focus is long-term shelf stability (e.g., archival materials, specialty food): The thoroughness of Secondary Drying is paramount to remove residual moisture and prevent degradation.
- If your primary focus is rapid reconstitution (e.g., instant coffee, injectable drugs): Controlling the Freezing stage to create larger ice crystals results in a more porous final product that rehydrates quickly.
By controlling these three stages, you can transform a perishable material into a stable, high-quality product with its core properties intact.
Summary Table:
| Stage | Key Process | Purpose |
|---|---|---|
| 1. Freezing | Solidify water below triple point | Create ice crystal structure for sublimation |
| 2. Primary Drying | Sublimation under vacuum | Remove ~95% of water as vapor |
| 3. Secondary Drying | Desorption of bound water | Achieve ultra-low moisture for stability |
Ready to optimize your freeze-drying process? KINTEK specializes in laboratory equipment and consumables, providing reliable freeze dryers and expert support to help you preserve delicate samples, pharmaceuticals, and specialty foods with precision. Contact our team today to discuss your specific application and ensure perfect results every time.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- Why is a freeze dryer preferred for reduced graphene oxide (Hh-RGO) powders? Preserve Nano-Structure and Performance
- What are the advantages of using freeze drying for phase change materials with biopolymer shells? Optimize Stability
- What is the function of Freeze-thaw Equipment in Au-(PNiPAAm/PVA) hydrogel? Achieve High-Speed Photothermal Actuation
- What role does a laboratory freeze dryer play in the synthesis of graphene-based electrocatalysts? Preserve 3D Structures
- Why is a laboratory vacuum freeze dryer essential for plant extracts? Preserve Bioactivity & Structure



















