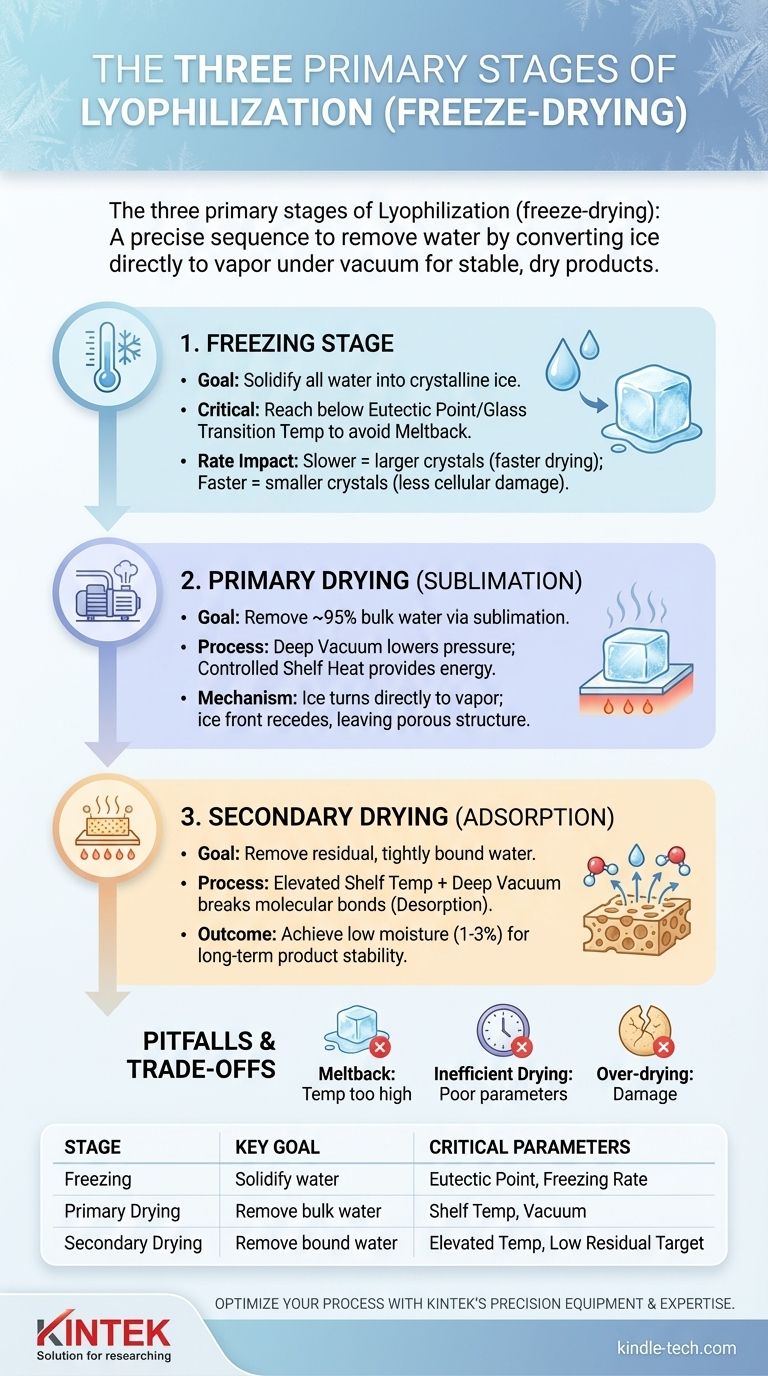
In short, a lyophilizer operates in three distinct stages: Freezing, Primary Drying (Sublimation), and Secondary Drying (Adsorption). These stages work sequentially to remove water from a product by first turning it to solid ice and then converting that ice directly into vapor under a deep vacuum, resulting in a stable, dry material.
The core challenge of lyophilization isn't just following three steps; it's about precisely managing the delicate balance between temperature and pressure. Mastering this interplay is the key to removing water without destroying the product's fundamental structure and integrity.

The Foundation: The Freezing Stage
The entire success of the lyophilization process is built upon a proper freezing stage. The goal is not just to make the product cold, but to convert all water into a solid, crystalline form, setting the stage for sublimation.
The Goal: Solidifying All Water
Before a vacuum can be pulled, the product must be cooled to a temperature where all freezable water turns to ice. This ensures that the water is removed via sublimation (solid to gas) rather than boiling (liquid to gas), which would destroy the product's structure.
Understanding the Critical Temperature
For simple substances, this is below the triple point. However, for complex mixtures (like most pharmaceuticals), the key threshold is the eutectic point or glass transition temperature. Freezing below this critical temperature is non-negotiable to prevent a catastrophic failure known as "meltback" during the drying phase.
The Impact of Freezing Rate
The speed at which a product is frozen dictates the size of the ice crystals. Slower freezing creates larger ice crystals, which form wider channels for water vapor to escape during drying, speeding up the process. Faster freezing creates smaller crystals, which can be less damaging to delicate cellular structures but may slow down the subsequent drying stage.
The Workhorse: Primary Drying (Sublimation)
This is the longest and most energy-intensive phase, where the bulk of the water (typically around 95%) is removed from the product.
Creating a Deep Vacuum
Once the product is properly frozen, the lyophilizer's vacuum pump reduces the chamber pressure significantly. This pressure drop is essential; it lowers the point at which ice will turn to vapor, allowing sublimation to occur at very low temperatures.
The Role of Controlled Heat
Sublimation is an endothermic process—it requires energy. The lyophilizer's shelves are gently heated, providing just enough thermal energy to the product to encourage the ice to turn into vapor. The product itself remains frozen due to the cooling effect of the sublimation process.
The Moving Sublimation Front
As ice sublimates, the "ice front" recedes through the product, leaving behind a porous, dry structure. The rate of sublimation is controlled by the balance between the vacuum level and the amount of heat applied via the shelves.
The Final Polish: Secondary Drying (Adsorption)
After all the free ice has been sublimated, a small amount of "bound" water remains, adsorbed to the molecules of the product itself. The secondary drying stage is designed to remove this residual moisture.
Targeting Bound Water
This water is much harder to remove than the free ice. It is ionically bonded to the product and requires more energy to be released.
How Temperature and Vacuum Work Together
To break these molecular bonds, the shelf temperature is raised significantly—often to well above 0°C—while the deep vacuum is maintained. This gives the remaining water molecules enough energy to escape the product, a process known as desorption.
Achieving Final Product Stability
The goal of this final stage is to reduce the residual moisture content to a target level, typically between 1% and 3%. This extremely low moisture content is what grants the final product its long-term stability at room temperature.
Understanding the Trade-offs and Pitfalls
A successful lyophilization cycle is a carefully optimized process. Misunderstanding the principles can lead to failed batches and damaged products.
Meltback: The Cardinal Sin of Lyophilization
If the product temperature rises above its critical eutectic temperature during primary drying, the frozen structure will collapse into a dense, gummy mass. This is an irreversible failure that ruins the product.
Inefficient Drying: The Cost of Poor Parameters
Using a shelf temperature that is too low or a vacuum that is not deep enough will drastically slow the rate of sublimation. This results in excessively long and inefficient cycle times, increasing operational costs.
Over-drying and Product Damage
While the goal of secondary drying is to remove bound water, applying too much heat can be destructive. Excessive temperatures can denature sensitive proteins or degrade other active pharmaceutical ingredients, compromising the final product's efficacy.
Making the Right Choice for Your Goal
Your process parameters should be tailored to your specific product and desired outcome.
- If your primary focus is preserving biological activity (e.g., vaccines, proteins): Prioritize precise temperature control to stay well below the critical temperature and avoid meltback at all costs.
- If your primary focus is maximum long-term stability: Concentrate on an effective secondary drying stage to achieve the lowest possible residual moisture content without thermally damaging the product.
- If your primary focus is optimizing cycle time and throughput: Invest in accurately determining your product's eutectic point to run the primary drying stage at the highest possible safe temperature.
Mastering lyophilization comes from understanding that it is a dynamic process of controlled energy transfer, not merely a static, three-step recipe.
Summary Table:
| Stage | Key Goal | Critical Parameters |
|---|---|---|
| 1. Freezing | Solidify all freezable water into ice | Eutectic point, Glass transition temperature, Freezing rate |
| 2. Primary Drying (Sublimation) | Remove ~95% of water via sublimation | Shelf temperature, Chamber pressure (vacuum) |
| 3. Secondary Drying (Adsorption) | Remove bound water for final stability | Elevated shelf temperature, Low residual moisture target |
Ready to Optimize Your Lyophilization Process?
Mastering the delicate balance of temperature and pressure is key to successful freeze-drying. Whether your goal is preserving sensitive biologics, achieving maximum product stability, or improving cycle throughput, KINTEK has the expertise and reliable lab equipment to support you.
We provide:
- Precision Lyophilizers for exact temperature and vacuum control.
- Expert Consultation to help you define critical parameters for your specific product.
- Durable Consumables to ensure consistent, reliable results batch after batch.
Let's discuss your laboratory's freeze-drying needs. Contact our experts today to find the perfect solution for your research or production goals.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Liquid Nitrogen Cryogenic Grinder Mill Cryomill Airflow Ultrafine Pulverizer
- Laboratory Vibratory Sieve Shaker Machine for Dry and Wet Three-Dimensional Sieving
People Also Ask
- What types of Laboratory Freeze Dryers are available and what are their applications? Choose the Right Lyophilizer for Your Lab
- What are the hazards of evaporators? Manage Chemical, Thermal, and Pressure Risks
- In what ways does freeze drying improve pharmaceutical product quality? Extend Shelf-Life and Preserve Drug Efficacy
- What are the three stages of freeze drying? A Guide to Lyophilization for Lab Professionals
- What property determines evaporation rate? Control heat, pressure, and surface area for optimal results.



















