In practice, pyrolysis reactions are classified into three primary methods based on the operational temperature: low, medium, and high. This classification is critical because temperature is the single most influential factor in determining the final output products of the process, whether they be solid, liquid, or gas.
The choice of pyrolysis temperature is not a minor detail; it is a strategic decision that dictates the chemical outcome. Lower temperatures favor the production of solid bio-char, while higher temperatures break down materials more completely to yield valuable bio-oils and flammable syngas.

The Fundamental Role of Temperature
Pyrolysis is the thermal decomposition of materials at elevated temperatures in an oxygen-deprived environment. Temperature acts as the engine of this transformation, controlling both the speed of the reaction and the types of molecules that are formed.
How Temperature Controls the Outcome
At lower temperatures, there is enough energy to break weaker chemical bonds, driving off volatile components but leaving much of the carbon backbone intact. This results in a high yield of solid bio-char.
As temperatures increase, more energy is available to crack larger organic molecules into smaller, condensable vapors. When cooled, these vapors form a liquid known as bio-oil.
At very high temperatures, the thermal cracking is so intense that almost all organic matter is broken down into the simplest, most stable gaseous molecules. This produces a mixture called syngas (synthesis gas).
A Breakdown of Pyrolysis Temperature Ranges
While exact figures vary based on feedstock and technology, the classifications provide a reliable framework for understanding the process and its expected products.
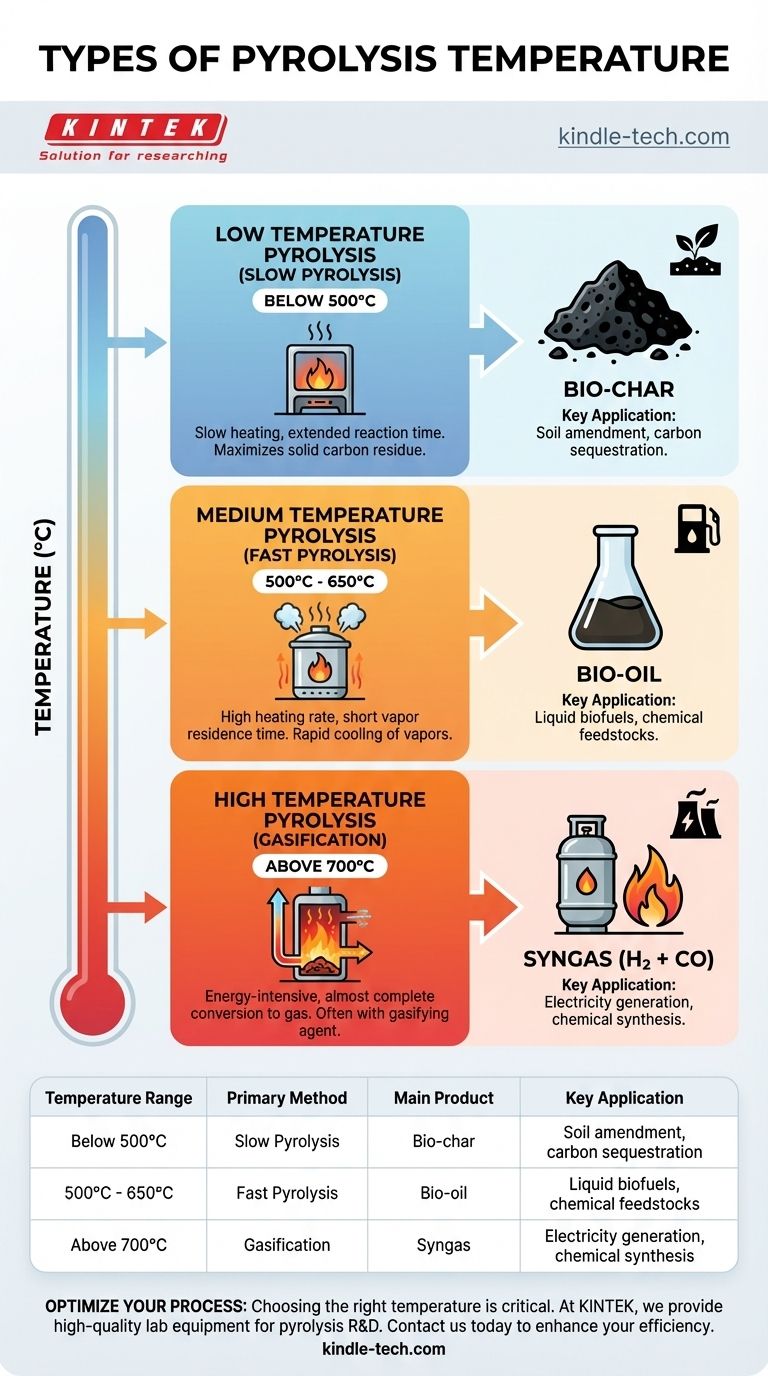
Low-Temperature Pyrolysis (Slow Pyrolysis)
Typically conducted at temperatures below 500°C, this process uses a slow heating rate. The extended reaction time and lower energy input maximize the production of a solid carbon residue.
The primary output is bio-char, a stable, carbon-rich material. This process is often favored for waste management and creating agricultural soil amendments.
Medium-Temperature Pyrolysis (Fast Pyrolysis)
This is the most common method for producing liquid fuels, operating in a range of roughly 500°C to 650°C. It requires a very high heating rate and short vapor residence time.
These conditions are optimized to break down biomass into vapors that, when rapidly cooled (quenched), produce the maximum yield of bio-oil. Bio-oil can be upgraded into transportation fuels or used to produce specialty chemicals.
High-Temperature Pyrolysis (Gasification)
Operating at temperatures above 700°C, this energy-intensive process aims to convert the feedstock almost entirely into gas. This is often referred to as gasification, especially when a gasifying agent like steam is introduced.
The main product is syngas, a mixture of hydrogen (H₂) and carbon monoxide (CO). Syngas is a versatile fuel that can be burned to generate electricity or used as a chemical building block for producing fuels and chemicals.
Understanding the Key Trade-offs
Choosing a temperature range involves a direct trade-off between energy input, operational complexity, and the value of the desired end product.
Energy vs. Product Value
High-temperature processes demand significantly more energy to maintain but produce high-value syngas, which is very energy-dense. Low-temperature pyrolysis is less energy-intensive but yields bio-char, a product with value in agriculture rather than as a high-density fuel.
Equipment and Complexity
Fast pyrolysis, used for bio-oil production, requires sophisticated reactors capable of extremely rapid heating and quenching. This increases capital and operational costs compared to the simpler reactors used for slow, low-temperature char production.
Making the Right Choice for Your Goal
Your target product should dictate the temperature and process you choose.
- If your primary focus is carbon sequestration or soil improvement: Use low-temperature (slow) pyrolysis to maximize the yield of stable bio-char.
- If your primary focus is creating a liquid biofuel or chemical feedstock: Use medium-temperature (fast) pyrolysis to optimize the production of bio-oil.
- If your primary focus is generating electricity or producing synthesis gas: Use high-temperature pyrolysis to convert the feedstock almost completely into valuable syngas.
Ultimately, temperature is the primary lever you can pull to steer the pyrolysis reaction toward the products that best meet your objective.
Summary Table:
| Temperature Range | Primary Method | Main Product | Key Application |
|---|---|---|---|
| Below 500°C | Slow Pyrolysis | Bio-char | Soil amendment, carbon sequestration |
| 500°C - 650°C | Fast Pyrolysis | Bio-oil | Liquid biofuels, chemical feedstocks |
| Above 700°C | Gasification | Syngas | Electricity generation, chemical synthesis |
Ready to optimize your pyrolysis process? Choosing the right temperature is critical for maximizing your yield of bio-char, bio-oil, or syngas. At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored for pyrolysis research and development. Whether you're scaling up from lab to pilot or need precise temperature control systems, our experts can help you select the perfect solution for your specific feedstock and product goals.
Contact us today to discuss how our solutions can enhance your pyrolysis efficiency and output. Get in touch now!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs



















