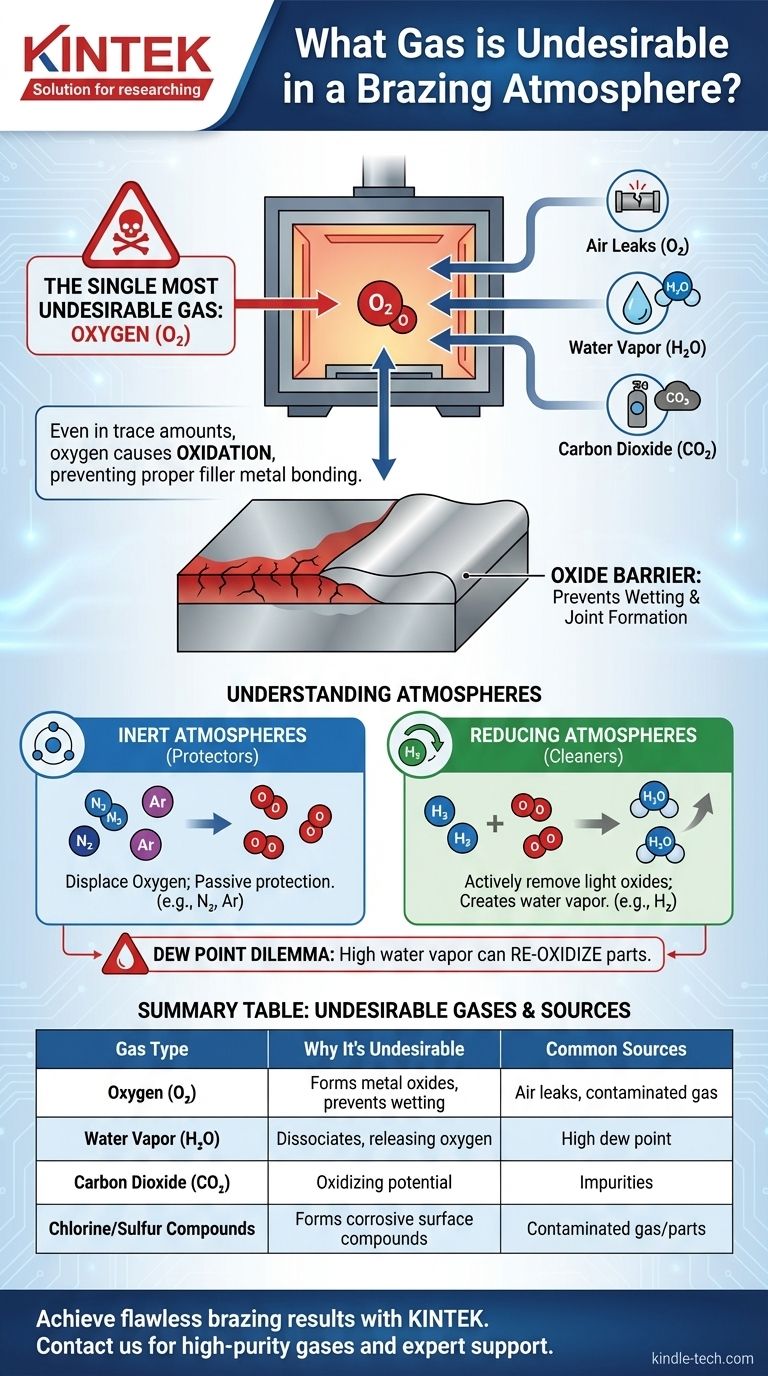
The single most undesirable gas in any brazing atmosphere is oxygen. Even in trace amounts, oxygen and gases that contain it—such as water vapor or carbon dioxide—are the primary cause of failed brazed joints because they form metal oxides at high temperatures, which prevent the filler metal from properly bonding to the parent materials.
The core purpose of a brazing atmosphere is to prevent the formation of oxides on the metal surfaces being joined. Therefore, any gas that introduces oxygen, directly or indirectly, fundamentally undermines the entire brazing process.

The Critical Role of the Brazing Atmosphere
Brazing relies on heating metals to a temperature where a filler metal can melt and flow into a joint via capillary action. The atmosphere within the furnace during this heating cycle is not passive; it is a critical variable that dictates the success or failure of the joint.
Preventing Oxidation
At elevated brazing temperatures, metals react very quickly with any available oxygen to form a thin, tenacious layer of metal oxide. This oxide layer acts as a barrier, preventing the molten brazing filler metal from making direct contact with the parent material. This phenomenon, known as poor "wetting," is a primary cause of weak or non-existent joints.
Promoting Filler Metal Flow
A clean, oxide-free surface is essential for the capillary action that pulls molten filler metal into the tight gaps of a joint. A proper brazing atmosphere protects the surfaces during heating, ensuring they remain pristine and allowing the filler metal to flow freely and uniformly throughout the entire joint.
The Primary Culprit: Oxygen and Its Sources
While pure oxygen is the obvious enemy, it often enters the brazing process from less direct sources. Controlling these is paramount for achieving a high-quality joint.
Free Oxygen (O₂)
This is the most direct contaminant. It can be introduced through leaks in the furnace, contaminated atmosphere gas, or insufficient purging of the furnace chamber before the heating cycle begins. Even a few parts per million (ppm) of oxygen can be enough to oxidize sensitive materials like stainless steel.
Water Vapor (H₂O)
Water vapor is a major, often underestimated, source of oxygen. At high brazing temperatures, water molecules can dissociate, releasing oxygen that readily forms oxides on the hot metal parts. The moisture content of an atmosphere gas, measured as its "dew point," is a critical parameter to monitor and control.
Carbon Dioxide (CO₂)
Similar to water vapor, carbon dioxide can also be an oxygen source at brazing temperatures. The CO₂ molecule can break down, creating an "oxidizing potential" that can be harmful to many common metals, particularly those containing chromium or other easily oxidized elements.
Other Reactive Gases
Gases like chlorine or sulfur compounds are also highly undesirable. While they don't necessarily form oxides, they are extremely corrosive and will aggressively react with the base metals. This creates other surface compounds (like chlorides) that also inhibit wetting and can lead to catastrophic post-braze corrosion or joint failure.
Understanding the Trade-offs: Inert vs. Reducing Atmospheres
Brazing atmospheres are generally classified as either inert or active (reducing). The choice depends on the materials being joined, the filler metal, and cost considerations.
Inert Atmospheres: The Protectors
Inert gases, such as Nitrogen (N₂) and Argon (Ar), work by simply displacing oxygen. They are passive protectors that create an environment where oxides cannot form. Nitrogen is a cost-effective workhorse for many applications, while higher-purity (and more expensive) Argon is used for highly sensitive materials like titanium.
Reducing Atmospheres: The Cleaners
An active or "reducing" atmosphere, typically containing Hydrogen (H₂), goes one step further. Hydrogen not only displaces oxygen but also actively removes light surface oxides by reacting with them to form water vapor (H₂O), which is then flushed from the furnace. This makes it excellent for cleaning parts that may have very light, pre-existing oxidation.
The Dew Point Dilemma
Using a hydrogen-rich atmosphere introduces a critical trade-off. While hydrogen cleans oxides by forming water vapor, that very same water vapor can re-oxidize the parts if its concentration becomes too high (a high dew point). A successful reducing atmosphere requires a careful balance where the water vapor produced is continuously removed, keeping the overall atmosphere dry.
Making the Right Choice for Your Goal
Selecting the correct atmosphere is a function of your materials, your quality requirements, and your budget.
- If your primary focus is cost-effective brazing of carbon steels: A standard nitrogen atmosphere is typically sufficient to prevent heavy oxidation and produce a quality joint.
- If your primary focus is brazing stainless steels, superalloys, or other sensitive metals: You must use a high-purity, very dry atmosphere like pure Argon or a Nitrogen/Hydrogen blend with a low dew point.
- If your primary focus is cleaning parts with light surface oxides during the cycle: A reducing atmosphere containing a percentage of Hydrogen is the ideal choice to ensure a pristine surface for wetting.
Ultimately, controlling the furnace atmosphere is the single most important factor in achieving consistent, high-integrity brazed joints.
Summary Table:
| Gas Type | Why It's Undesirable | Common Sources |
|---|---|---|
| Oxygen (O₂) | Directly forms metal oxides, preventing filler metal wetting. | Air leaks, contaminated gas, insufficient purging. |
| Water Vapor (H₂O) | Dissociates at high temperature, releasing oxygen. | High dew point in atmosphere gas. |
| Carbon Dioxide (CO₂) | Can break down and provide an oxidizing potential. | Impurities in atmosphere gas. |
| Chlorine/Sulfur Compounds | Forms corrosive surface compounds that inhibit wetting. | Contaminated gas or parts. |
Achieve flawless brazing results with KINTEK.
Preventing oxidation is the key to strong, reliable brazed joints. Whether you are brazing carbon steel, sensitive stainless steels, or superalloys, selecting and controlling the right furnace atmosphere is critical.
KINTEK specializes in providing the high-purity gases and expert support your laboratory needs to maintain perfect brazing atmospheres. We help you eliminate undesirable gases and ensure consistent, high-integrity results.
Contact us today to discuss your brazing application and how we can support your success. Get in touch via our contact form
Visual Guide
Related Products
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
People Also Ask
- What is an example of an inert atmosphere? Discover the Best Gas for Your Process
- What is the purpose of inert atmosphere? A Guide to Protecting Your Materials and Processes
- What is meant by inert atmosphere? A Guide to Preventing Oxidation & Ensuring Safety
- Why nitrogen is used in furnace? A Cost-Effective Shield for High-Temperature Processes
- What provides an inert atmosphere? Achieve Safety and Purity with Nitrogen, Argon, or CO2



















