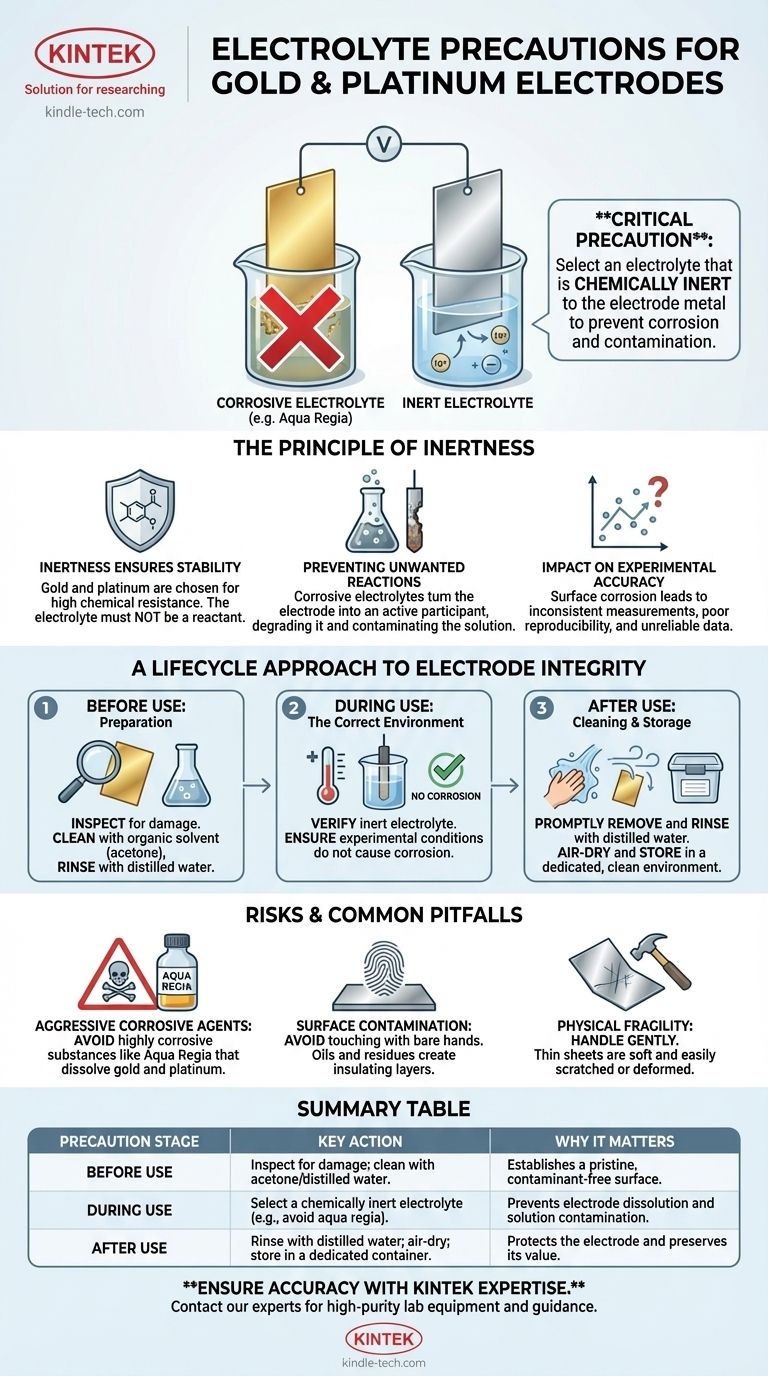
The most critical precaution when using gold or platinum sheet electrodes is to select an electrolyte that will not chemically react with or corrode the metal. These noble metals are chosen for their stability, and using an inappropriate electrolyte can dissolve the electrode, contaminate your experiment, and render your results invalid. A classic example of a substance to avoid is aqua regia, which is known to dissolve gold.
The core principle is not just about the electrolyte, but about maintaining the absolute chemical purity and physical integrity of the electrode surface. Any contamination or reaction—whether from the electrolyte, handling, or storage—will fundamentally compromise the accuracy and reliability of your work.

The Principle of Inertness: Why Your Choice Matters
Gold and platinum are used in high-precision applications because they are chemically stable, or inert. The goal is for the electrode to provide a stable surface for the desired electrochemical reaction to occur, not to become a reactant itself.
Preventing Unwanted Reactions
The high purity of these sheets, typically 99.99%, is what ensures their stable performance. Introducing an electrolyte that attacks the metal undermines this fundamental property. Your electrode goes from being a stable platform to an active, and unwanted, participant in the chemical process.
The Role of the Electrolyte
The electrolyte's job is to conduct ions and facilitate the intended reaction at the electrode's surface. If the electrolyte is corrosive, it creates a competing, destructive reaction that degrades the electrode. This not only ruins the expensive material but also introduces metallic ions into your solution, contaminating the entire experiment.
Impact on Experimental Accuracy
Even minor surface corrosion or contamination can dramatically alter the electrochemical properties of the electrode. This leads to inconsistent measurements, poor reproducibility, and unreliable data. In high-precision work, a pristine surface is non-negotiable.
A Lifecycle Approach to Electrode Integrity
Protecting your electrode isn't a single action, but a continuous process that spans before, during, and after the experiment.
Before Use: Preparation and Inspection
Always inspect the sheet's surface for physical damage like scratches, pits, or deformation. Minor flaws can significantly impact performance.
If you suspect surface contamination, clean the sheet with an organic solvent like acetone to remove oils, then rinse it thoroughly with distilled water. This establishes a clean baseline for your experiment.
During Use: The Correct Environment
This is where the choice of a non-reactive electrolyte is paramount. Verify that your chosen electrolyte and experimental conditions (like temperature and voltage) will not cause the gold or platinum to corrode or dissolve.
After Use: Cleaning and Storage
Promptly remove the sheet from the electrolyte after the experiment concludes. Rinse it repeatedly with distilled water to remove any residual substances.
Allow the sheet to air-dry completely in a clean, dust-free environment. Store it in a dedicated, dry container where it will not be scratched or come into contact with other materials that could cause contamination.
Understanding the Risks and Common Pitfalls
While robust, these materials are not indestructible. Awareness of their vulnerabilities is key to their longevity and proper function.
The Danger of Aggressive Corrosive Agents
Never allow gold or platinum sheets to come into contact with highly corrosive substances. Aqua regia, a mixture of concentrated nitric and hydrochloric acids, is the most well-known agent that will actively dissolve gold and damage platinum.
The Threat of Surface Contamination
Avoid touching the electrode surface with bare hands or letting it contact other organic materials. Oils and residues can create an insulating layer that interferes with electrochemical measurements.
The Physical Fragility of the Sheets
These sheets are often very thin (0.1mm to 0.5mm) and made of soft metals. Handle them gently to prevent scratches, bends, or deformation. Physical damage is difficult to repair and permanently alters the electrode's surface area and performance characteristics.
Making the Right Choice for Your Goal
Your handling protocol should directly support your primary objective.
- If your primary focus is experimental accuracy: Your first step must be to verify that your chosen electrolyte is chemically inert with respect to gold or platinum under your specific experimental conditions.
- If your primary focus is long-term asset preservation: Implement a strict protocol for immediate post-experiment cleaning, gentle handling, and isolated storage to prevent both chemical corrosion and physical damage.
- If you are troubleshooting inconsistent results: Re-evaluate your entire handling process, from pre-experiment cleaning with solvents to post-experiment rinsing, as subtle surface contamination is a frequent cause of error.
By treating your electrode's surface purity as a non-negotiable prerequisite, you ensure the validity of your work and the longevity of your investment.
Summary Table:
| Precaution Stage | Key Action | Why It Matters |
|---|---|---|
| Before Use | Inspect for damage; clean with acetone/distilled water. | Establishes a pristine, contaminant-free surface. |
| During Use | Select a chemically inert electrolyte (e.g., avoid aqua regia). | Prevents electrode dissolution and solution contamination. |
| After Use | Rinse with distilled water; air-dry; store in a dedicated container. | Protects the electrode for future experiments and preserves its value. |
Ensure the accuracy and longevity of your experiments with the right equipment and expertise. KINTEK specializes in high-purity lab equipment and consumables, including gold and platinum electrodes designed for stability and precision. Our team can help you select the perfect materials for your specific electrochemical applications. Contact our experts today to discuss your lab's needs and discover how we can support your research integrity.
Visual Guide
Related Products
- Gold Electrochemical Sheet Electrode Gold Electrode
- Gold Disc Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Metal Disc Electrode Electrochemical Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
People Also Ask
- How should a gold plate electrode be handled during an experiment? Ensure Accurate and Reproducible Results
- What is the operating principle of a gold disc electrode in an electrochemical system? Unlock Precision with a Stable Interface
- What precautions should be taken to prevent mechanical damage to a gold plate electrode? Protect Your Data Integrity
- What are the key aspects of maintaining and caring for a gold plate electrode? Preserve Performance and Extend Lifespan
- How should contamination of a gold plate electrode be prevented and managed? Essential Care for Reliable Data



















