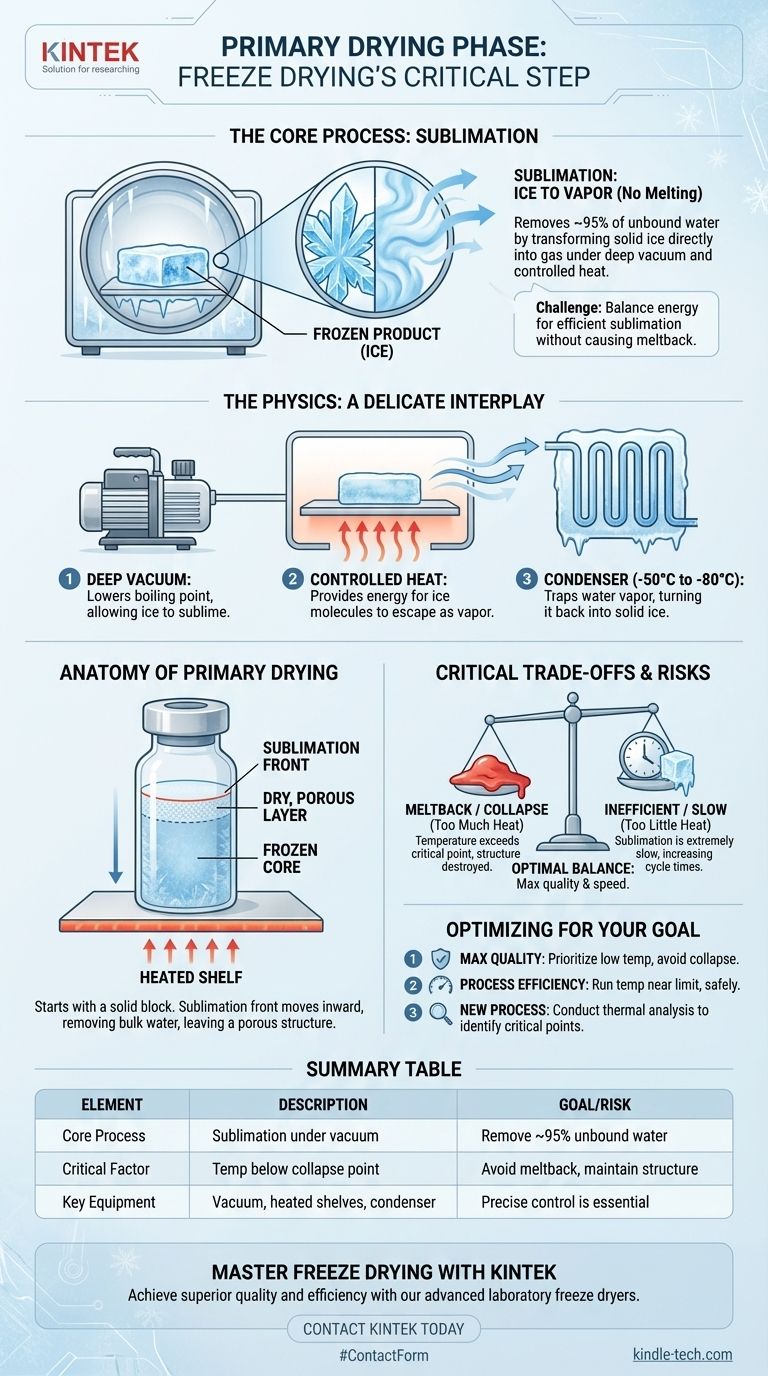
In the simplest terms, the primary drying phase of freeze drying removes frozen water from a material not by melting it, but by transforming it directly from a solid (ice) into a gas (vapor). This process, known as sublimation, is achieved by placing the frozen product under a deep vacuum while carefully adding a controlled amount of heat. This critical stage is responsible for removing the vast majority of water—typically around 95%—from the product.
The central challenge of primary drying is a delicate balance: applying just enough energy to drive sublimation efficiently without raising the product's temperature to its melting point, which would irreversibly destroy its structure.

The Physics of Sublimation: Removing Water Without Melting
To understand primary drying, you must first understand the unique conditions required to make ice behave like a gas instead of a liquid. The process is governed by a precise interplay of pressure and temperature.
Creating a Deep Vacuum
The first step is to drastically lower the pressure inside the freeze dryer chamber. This vacuum is essential because it lowers the temperature at which water boils. Under a deep vacuum, ice no longer needs to become liquid water before it can turn into vapor.
The Role of Controlled Heat
Sublimation is an energy-intensive process; the ice needs energy to transform into vapor. This energy is supplied by gently heating the shelves on which the product sits. This heat provides the fuel for the ice molecules to escape directly into the gaseous state.
Capturing the Water Vapor
As water vapor leaves the product, it must be removed from the chamber to maintain the low pressure. This is the job of the condenser, an extremely cold surface (often -50°C to -80°C) within the freeze dryer. The water vapor instantly freezes back into solid ice on the condenser coils, effectively trapping it and keeping the system's pressure low.
The Anatomy of the Primary Drying Phase
This phase is the longest and most critical part of the entire freeze-drying cycle, defining the structure and quality of the final product.
Starting with a Solid Block
Before primary drying can begin, the material must be completely and solidly frozen. This initial freezing stage creates the ice crystal structure that will become the porous, sponge-like architecture of the dried product.
The Moving "Sublimation Front"
As drying progresses, a boundary known as the sublimation front moves through the product. Ice sublimes from the outer surface first, leaving behind a dry, porous layer. This front slowly recedes deeper into the product until all the unbound ice has been converted to vapor.
Removing the Bulk of Water
This single phase is responsible for removing all the "free" or unbound water in the product. Because it involves a phase change for a massive amount of water, it is by far the most time-consuming part of the freeze-drying process.
Understanding the Critical Trade-offs
The success of primary drying hinges on managing one key risk: adding too much heat too quickly.
The Risk of "Meltback" or "Collapse"
Every product has a critical temperature (often related to its eutectic point) below which it must remain during primary drying. If the product temperature rises above this point, the ice will melt instead of sublimating. This liquid water destroys the carefully created porous structure, a catastrophic failure known as "collapse" or "meltback."
The Cost of Being Too Cautious
Conversely, applying too little heat makes the sublimation process extremely slow and inefficient. This can dramatically increase cycle times and energy costs, making the process commercially unviable. The goal is always to run the cycle as warm as possible without risking a collapse.
The Importance of Pressure Control
The vacuum level must also be carefully controlled. If the pressure is too high, it can contribute to melting. If it is too low, the rate of heat transfer to the product can slow down, unnecessarily extending the drying time.
Optimizing Primary Drying for Your Goal
Success in freeze drying hinges on tailoring the primary drying parameters to the specific thermal characteristics of your material.
- If your primary focus is maximum product quality and structural integrity: Prioritize keeping the product temperature well below its critical collapse temperature, even if it significantly extends the drying time.
- If your primary focus is process efficiency and speed: Carefully determine the product's maximum allowable temperature through analysis and run the shelf temperature as close to that limit as is safely possible.
- If you are developing a new process for a sensitive material: Conduct thorough thermal analysis to precisely identify your product's critical temperature before attempting to scale up the process.
Mastering this delicate phase is the absolute key to creating a stable, high-quality, and easily rehydrated final product.
Summary Table:
| Primary Drying Phase Key Elements | Description |
|---|---|
| Core Process | Sublimation: Ice transforms directly to vapor under vacuum. |
| Main Goal | Remove ~95% of unbound (free) water from the frozen product. |
| Critical Factor | Product temperature must stay below its collapse/melting point. |
| Key Equipment | Vacuum chamber, heated shelves, and a cold condenser (e.g., -50°C to -80°C). |
| Primary Risk | Applying too much heat causes meltback/collapse, destroying product structure. |
Optimize Your Freeze-Drying Process with KINTEK
Mastering the delicate balance of heat and vacuum during primary drying is critical for producing high-quality, stable lyophilized products. Whether you are developing a new pharmaceutical formulation or preserving sensitive food materials, the right equipment is essential for success.
KINTEK specializes in advanced laboratory freeze dryers and consumables designed for precise control and reliability. Our solutions help you:
- Achieve Superior Product Quality: Maintain precise temperatures to prevent collapse and ensure structural integrity.
- Increase Process Efficiency: Optimize cycle times with controlled heating and vacuum systems.
- Scale with Confidence: From R&D to production, our equipment supports your entire development workflow.
Let our experts help you select the perfect freeze-drying system for your specific application. Contact us today to discuss your laboratory needs and request a consultation.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- Why is a freeze dryer preferred for drying nickel nanoparticle precursors? Prevent Hard Agglomeration Now
- What is the purpose of an evaporator? The Key Component That Creates Cooling
- Why is a freeze dryer preferred over thermal drying for Fe-ZTA cermets? Ensure Pure, Homogeneous Slurry Processing
- Why is a laboratory freeze-drying system essential for fermentation biomass? Preserve Sample Integrity for Analysis
- Why is a freeze dryer preferred for reduced graphene oxide (Hh-RGO) powders? Preserve Nano-Structure and Performance



















