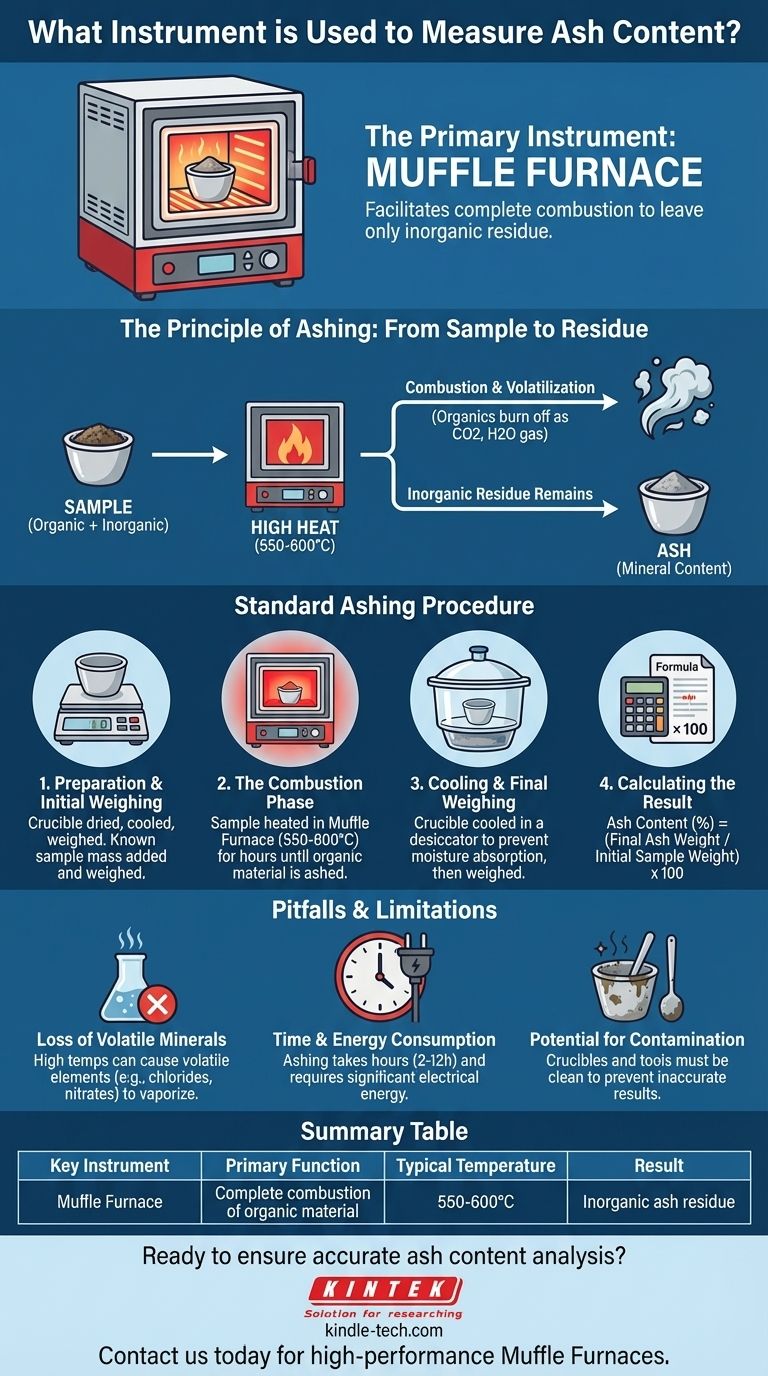
The primary instrument used to measure ash content is a muffle furnace. This is a high-temperature oven designed to completely burn away the organic material in a sample, a process known as incineration or "ashing." The remaining inorganic, noncombustible residue is the ash, which is then weighed to determine the ash content.
The key is to understand that ash content isn't "measured" directly by an instrument. Instead, a muffle furnace facilitates a process of complete combustion, and the final ash content is determined by weighing the mineral residue that remains.

The Principle of Ashing: From Sample to Residue
To truly understand the role of the muffle furnace, you must first understand the principle behind determining ash content. It's a method of separation by thermal decomposition.
What is Ash Content?
Ash is the inorganic residue that remains after the complete combustion of a sample. It represents the total mineral content, which typically consists of oxides of elements like calcium, potassium, magnesium, and phosphorus.
The Goal of Complete Combustion
The entire purpose of the ashing process is to eliminate all organic matter—compounds based on carbon, hydrogen, and oxygen. By heating a sample to very high temperatures (typically 550-600°C), these organic components are burned off and converted into gases like carbon dioxide and water vapor.
How a Muffle Furnace Works
A muffle furnace is essentially a specialized laboratory oven capable of reaching and maintaining these high, uniform temperatures. Its name comes from the "muffle," an insulating chamber that separates the sample from the heating elements. This ensures that the sample is heated evenly through convection and radiation, not by direct flame, leading to a consistent and complete burn.
The Standard Ashing Procedure
While specific protocols vary by industry (e.g., food, materials science, environmental testing), the fundamental steps performed with a muffle furnace are consistent.
Step 1: Preparation and Initial Weighing
A heat-resistant crucible, typically made of porcelain, is heated to remove any moisture, cooled, and weighed precisely. A known mass of the sample is then placed in the crucible and weighed again.
Step 2: The Combustion Phase
The crucible containing the sample is placed inside the pre-heated muffle furnace. It is left there for several hours until all the organic material has turned to ash, which usually appears as a light gray or white powder.
Step 3: Cooling and Final Weighing
The crucible is carefully removed from the furnace and placed in a desiccator. A desiccator is a sealed container with a drying agent that prevents the ash from absorbing moisture from the air while it cools. Once at room temperature, the crucible with the ash is weighed a final time.
Step 4: Calculating the Result
The calculation is straightforward: the weight of the final ash is divided by the initial weight of the sample, then multiplied by 100 to yield the ash content percentage.
Understanding the Pitfalls and Limitations
Using a muffle furnace for "dry ashing" is the most common method, but it's essential to recognize its limitations.
Loss of Volatile Minerals
The extremely high temperatures can cause certain volatile mineral elements and compounds to be lost. For example, chlorides and nitrates can vaporize and escape, leading to an underestimation of the true total mineral content.
Time and Energy Consumption
The ashing process is not quick. It can take anywhere from two to twelve hours, depending on the sample type and size. Muffle furnaces also consume a significant amount of electrical energy to maintain their high temperatures.
Potential for Contamination
Care must be taken to use clean crucibles and tools. Any foreign material introduced before or after combustion will lead to inaccurate weight measurements and flawed results.
Making the Right Choice for Your Goal
The method for determining ash content depends entirely on what you need to learn from the analysis.
- If your primary focus is routine quality control: The muffle furnace (dry ashing) is the standard, most reliable method for determining the total inorganic content as a key quality parameter.
- If your primary focus is analyzing for specific volatile minerals (like lead or mercury): You must consider alternative methods like wet ashing, which uses acids to digest the sample at lower temperatures, preventing these elements from being lost.
- If your primary focus is a simple pass/fail on material purity: A muffle furnace provides a straightforward and cost-effective way to check if a material's inorganic filler content is within specification.
Ultimately, the muffle furnace is the foundational tool for revealing the noncombustible, mineral-based backbone of a material.
Summary Table:
| Key Instrument | Primary Function | Typical Temperature | Result |
|---|---|---|---|
| Muffle Furnace | Complete combustion of organic material | 550-600°C | Inorganic ash residue |
Ready to ensure accurate and reliable ash content analysis in your lab?
KINTEK specializes in high-performance muffle furnaces and lab equipment designed for precise thermal applications like ashing. Our solutions provide the uniform heating and temperature control essential for consistent, reproducible results in quality control, food testing, and materials science.
Contact us today to find the perfect furnace for your specific needs and enhance your laboratory's capabilities.
Get in touch with our experts now!
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What are the working principles of furnace? A Guide to Combustion, Resistance, and Induction Heating
- Is a muffle furnace used for ash determination? Discover Its Critical Role in Accurate Analysis
- How does a muffle furnace work? A Guide to Clean, High-Temperature Heating
- What temperature does steel liquify? Understanding the Melting Range for Your Applications
- What are the applications of muffle furnaces? Essential Tools for High-Temperature Processes



















