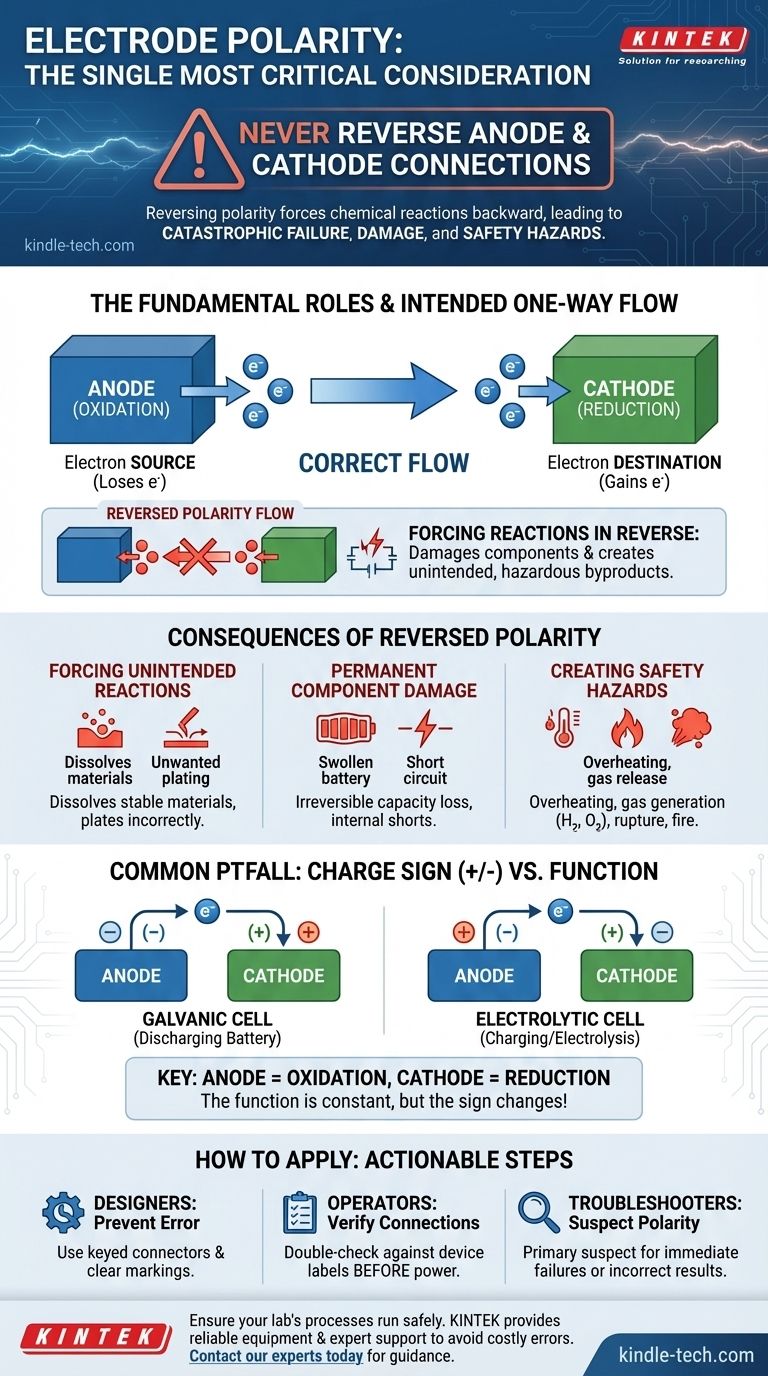
The single most critical consideration for electrode polarity is that the anode and cathode connections must never be reversed. This is not merely a convention; reversing polarity can force chemical reactions to run backward, leading to immediate and often irreversible damage to the components, incorrect system function, or even significant safety hazards.
Electrode polarity dictates the fundamental direction of the intended chemical reaction. Reversing it is akin to forcing an engine to run in reverse—it actively works against the system's design, leading to catastrophic failure rather than the desired outcome.

The Fundamental Roles of Anode and Cathode
To understand why reversing polarity is so damaging, we must first establish the specific and non-interchangeable roles of the two electrodes.
The Anode: Site of Oxidation
The anode is defined as the electrode where oxidation occurs. This is the process where a chemical species loses electrons.
Think of the anode as the "source" in the electrical circuit, releasing electrons into the system as a result of the chemical reaction.
The Cathode: Site of Reduction
The cathode is the electrode where reduction occurs. This is the complementary process where a chemical species gains the electrons that the anode released.
The cathode acts as the "destination," consuming electrons to complete the chemical reaction and the electrical circuit.
Why This Direction Matters
The entire electrochemical system—whether it's a battery, a sensor, or an electroplating bath—is designed around this specific, one-way flow of electrons from anode to cathode. The materials for each electrode are chosen precisely for their ability to perform either oxidation or reduction efficiently.
The Consequences of Reversed Polarity
Connecting the anode and cathode incorrectly forces electricity to flow in the wrong direction, compelling each electrode to perform a chemical function it was not designed for.
Forcing Unintended Reactions
When polarity is reversed, you apply a voltage that attempts to make the cathode oxidize and the anode reduce. This can dissolve materials that should be stable or plate materials onto surfaces where they don't belong, creating unwanted chemical byproducts.
Permanent Component Damage
In a rechargeable battery, for example, reversing the connection during charging can cause lithium metal to plate onto the anode, permanently reducing the battery's capacity and creating an internal short circuit risk. In electroplating, it would begin dissolving the very object you intend to coat.
Creating Safety Hazards
Forcing the wrong reactions can lead to dangerous outcomes. Overheating is common, and in aqueous systems, the breakdown of water can rapidly generate flammable hydrogen and oxygen gas. In high-power applications like battery systems, this can lead to swelling, rupture, or fire.
Common Pitfalls to Avoid
The definition of anode and cathode is constant, but their charge sign (+ or -) can be a point of confusion because it depends on the type of electrochemical cell.
Galvanic vs. Electrolytic Cells
In a galvanic cell (one that produces energy, like a discharging battery), the anode is the negative terminal. The spontaneous oxidation reaction pushes electrons out.
In an electrolytic cell (one that consumes energy, like a charging battery or for electrolysis), the anode is the positive terminal. An external power source pulls electrons from it to drive a non-spontaneous reaction.
It is crucial to recognize that while the sign can change, the function does not: the anode is always where oxidation happens.
The Importance of Clear Labeling
Because of this potential for confusion, you must rely on the system's labels (+/-) and documentation. The designers have accounted for the cell type. Never assume the anode is always negative or positive without knowing the context.
How to Apply This to Your Project
Your approach to polarity depends on your role in the system's lifecycle.
- If your primary focus is designing a system: Your goal is to prevent user error. Use keyed connectors that can only be plugged in one way and provide clear, permanent markings for
+and-terminals. - If your primary focus is operating equipment: Your goal is verification. Always double-check connections against device markings or schematics before applying power, making it a critical step in any checklist.
- If your primary focus is troubleshooting a failure: Your goal is diagnosis. Reversed polarity should be a primary suspect if a component fails immediately upon connection or a process yields unexpected and incorrect results.
Properly respecting electrode polarity is the foundation for any safe, efficient, and functional electrochemical system.
Summary Table:
| Aspect | Correct Polarity | Reversed Polarity |
|---|---|---|
| Anode Function | Oxidation (loses electrons) | Forced to perform reduction |
| Cathode Function | Reduction (gains electrons) | Forced to perform oxidation |
| System Outcome | Designed, efficient operation | Catastrophic failure, damage |
| Safety Risk | Low (when operated correctly) | High (overheating, gas generation, fire) |
Ensure your lab's electrochemical processes run safely and efficiently. Incorrect electrode polarity can lead to immediate equipment damage, dangerous safety hazards, and ruined experiments. KINTEK specializes in providing reliable lab equipment and consumables, backed by expert technical support to help you avoid costly errors. Don't risk your research or safety—contact our experts today for guidance on selecting and operating the right electrochemical systems for your laboratory needs.
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Gold Disc Electrode
- Electrolytic Electrochemical Cell for Coating Evaluation
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- What optical features does the H-type electrolytic cell have? Precision Quartz Windows for Photoelectrochemistry
- What is a H type cell? A Guide to Divided Electrochemical Cells for Accurate Experiments
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide



















