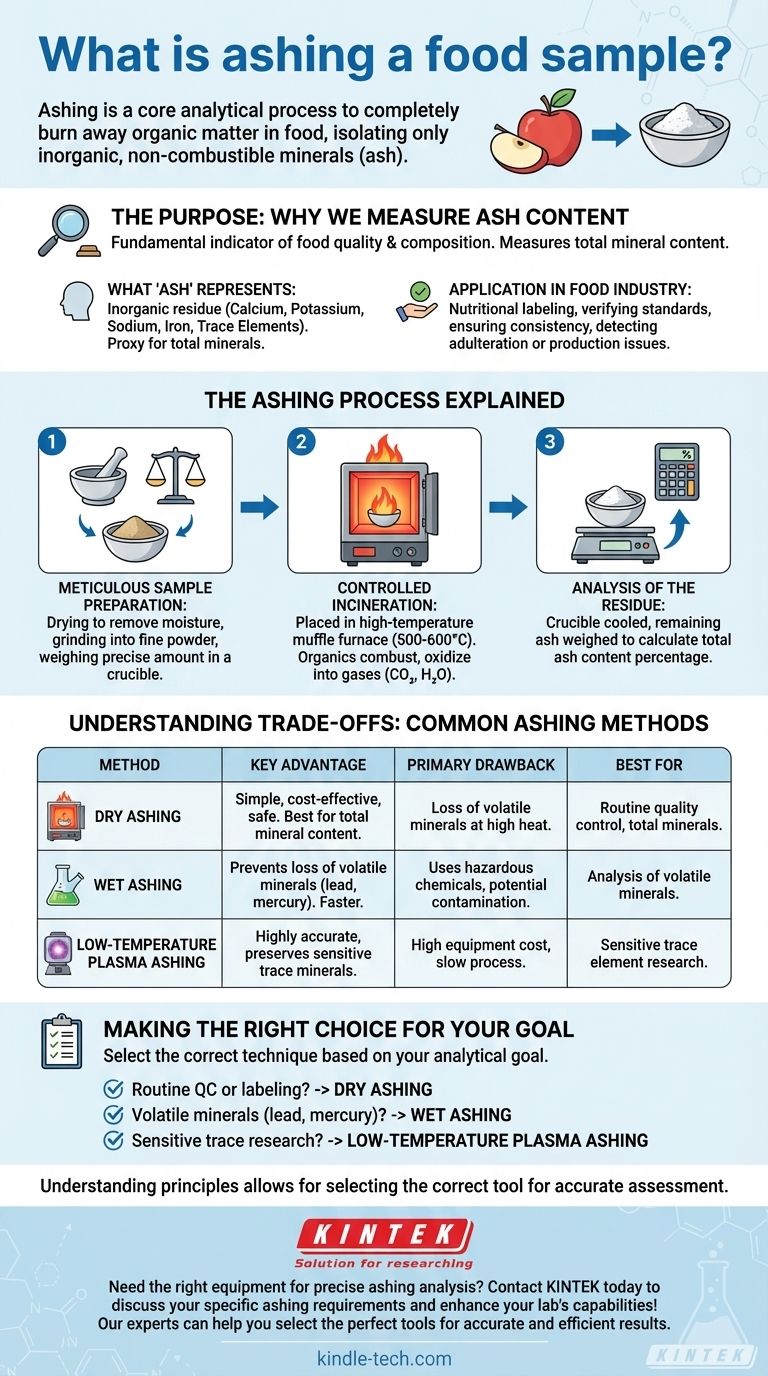
Ashing a food sample is a core analytical process used to completely burn away all the organic matter in a food product. This controlled incineration leaves behind only the inorganic, non-combustible components—the ash—which represents the food's total mineral content.
Ashing is not about destruction; it is about isolation. By incinerating the organic components (fats, proteins, carbohydrates), analysts can precisely measure the remaining inorganic minerals, a critical step for nutritional analysis and quality control.

The Purpose: Why We Measure Ash Content
The measurement of ash is a fundamental indicator of food quality and composition. It serves as a direct measure of the total amount of minerals present.
What "Ash" Represents
The ash that remains after the process is the inorganic residue. This includes essential minerals like calcium, potassium, sodium, and iron, as well as trace elements.
Essentially, ash content is the portion of the food that would not burn or evaporate. It's a proxy for the overall mineral content.
Application in the Food Industry
Food scientists and quality assurance teams measure ash for several key reasons. It is a crucial parameter for nutritional labeling, verifying food standards, and ensuring product consistency. Unusually high or low ash content can indicate adulteration or problems in the production process.
The Ashing Process Explained
The procedure is a carefully controlled and precise laboratory method designed to yield accurate results. It moves from sample preparation to complete combustion.
Step 1: Meticulous Sample Preparation
The sample must be prepared correctly to ensure accurate results. This involves drying the sample to remove all moisture, which prevents spattering during heating.
The dried sample is then ground into a fine powder to ensure it burns evenly and completely. A precise weight, typically between 1 and 10 grams, is measured into a special crucible that can withstand extreme temperatures.
Step 2: Controlled Incineration
The prepared sample is placed in a high-temperature muffle furnace. The temperature is gradually increased, causing the organic compounds to react with oxygen and combust.
This process oxidizes all carbohydrates, proteins, fats, and other organic materials, converting them into gases (like carbon dioxide and water vapor) that leave the sample.
Step 3: Analysis of the Residue
After a set period at high temperature (often several hours), all organic material is gone. The crucible is carefully removed from the furnace and cooled. The remaining ash is then weighed, allowing for the calculation of the total ash content as a percentage of the original sample's weight.
Understanding the Trade-offs: Common Ashing Methods
The choice of method depends on the specific minerals being analyzed and the required accuracy. There is no single "best" method for all applications.
Dry Ashing
This is the most common method, using a muffle furnace at high temperatures (500–600°C). It is simple, safe, and effective for measuring total mineral content.
However, its primary drawback is the potential loss of volatile minerals like lead, zinc, and selenium, which can vaporize and escape at high temperatures.
Wet Ashing
Wet ashing uses strong acids and oxidizing agents (like nitric and sulfuric acid) to digest the organic matter at much lower temperatures than dry ashing.
This method is faster and prevents the loss of volatile minerals. However, it requires handling hazardous chemicals, introduces potential for reagent contamination, and is not ideal for determining total ash content.
Low-Temperature Plasma Ashing
This advanced method uses a vacuum chamber and excited oxygen gas (plasma) to oxidize the sample at very low temperatures (below 150°C).
It is the most accurate method for preserving volatile trace minerals. Its major trade-offs are the high cost of equipment and the significantly longer time required for analysis.
Making the Right Choice for Your Goal
Selecting the correct ashing technique is critical for obtaining meaningful data. Your analytical goal should dictate your choice.
- If your primary focus is routine quality control or general nutritional labeling: Dry ashing is the standard, most cost-effective method for determining total mineral content.
- If your primary focus is analyzing for volatile minerals like lead or mercury: Wet ashing is necessary to prevent these elements from being lost at high temperatures.
- If your primary focus is highly sensitive trace element research: Low-temperature plasma ashing provides the most accurate results, though it demands specialized equipment and expertise.
Understanding the principles of ashing allows you to select the correct analytical tool to accurately assess the nutritional foundation of any food product.
Summary Table:
| Method | Key Advantage | Primary Drawback | Best For |
|---|---|---|---|
| Dry Ashing | Simple, cost-effective, safe | Loss of volatile minerals | Routine quality control, total mineral content |
| Wet Ashing | Prevents loss of volatile minerals | Uses hazardous chemicals | Analysis of volatile minerals (e.g., lead, mercury) |
| Low-Temperature Plasma Ashing | Highly accurate, preserves trace minerals | High equipment cost, slow process | Sensitive trace element research |
Need the right equipment for precise ashing analysis?
Choosing the correct method is crucial for accurate results. KINTEK specializes in high-quality lab equipment, including reliable muffle furnaces for dry ashing and consumables for all your laboratory needs. Our experts can help you select the perfect tools to ensure your food quality control and nutritional labeling are accurate and efficient.
Contact KINTEK today to discuss your specific ashing requirements and enhance your lab's capabilities!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the methods of ashing food? Choose the Right Technique for Accurate Mineral Analysis
- What is the function of sintering? Transforming Powder into Strong, Solid Components
- How does a constant temperature drying oven facilitate the CBD process of a SnO2 ETL? Optimize Your Film Morphology
- What is the specific industrial function of an ashing or tempering furnace for MgO-C refractories? Curing for Strength
- What is the concept of muffle furnace? Achieve Clean, Uniform High-Temperature Processing
- Which furnace is used for heat treatment of small parts? Select the Right Tool for Your Process
- What is the difference between annealed and tempered steel? Master the Heat Treatment Process
- How does a temperature-controlled heat treatment furnace balance hardness and toughness? Achieve Material Excellence



















