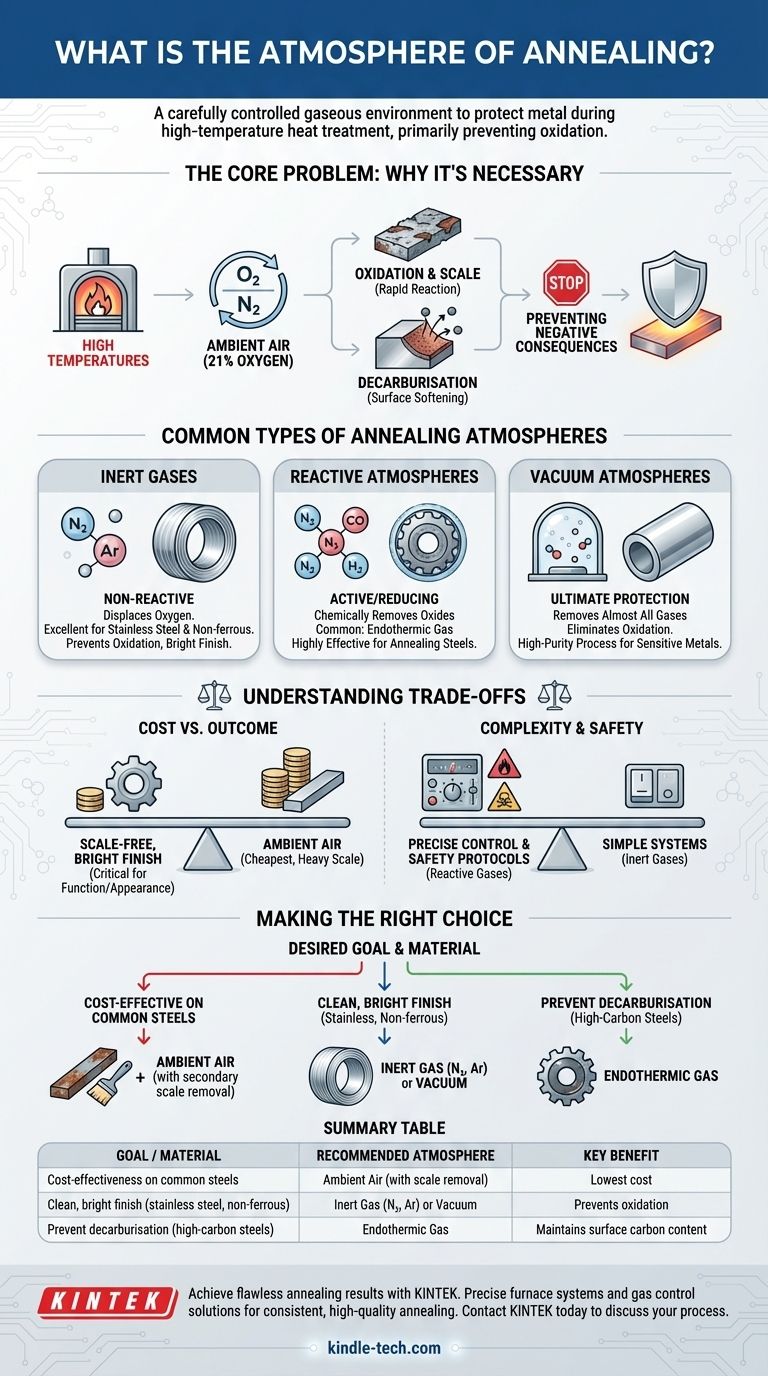
The atmosphere of annealing refers to the carefully controlled gaseous environment within a furnace during the heat treatment process. Its purpose is to protect the metal from undesirable chemical reactions with the surrounding air, most notably oxidation, which occurs rapidly at high temperatures. Common atmospheres include inert gases, specific gas mixtures like endothermic gas, or a near-vacuum.
The core function of a controlled annealing atmosphere is not to enable the heat treatment, but to prevent a negative consequence. By displacing oxygen, a protective atmosphere acts as a shield for the hot metal surface, preventing the formation of scale and ensuring the material's surface integrity and properties are preserved.

The Core Problem: Why a Controlled Atmosphere is Necessary
Annealing requires high temperatures, which greatly accelerate chemical reactions. Exposing hot metal to ambient air, which contains about 21% oxygen, creates significant challenges that a controlled atmosphere is designed to solve.
The Threat of Oxidation and Scale
The most immediate problem is oxidation. At annealing temperatures, the metal's surface will rapidly react with oxygen to form a layer of metallic oxide, commonly known as scale.
This scale is often undesirable as it alters the surface finish, can flake off, and may need to be removed through costly secondary processes like acid pickling or sandblasting.
The Risk of Decarburisation
For high-carbon steels, another risk is decarburisation. This is a process where carbon atoms at the surface of the steel react with the atmosphere and are lost.
Losing carbon from the surface layer effectively softens it, which can compromise the performance of the final component, especially if that surface needs to be hard and wear-resistant.
Ensuring Consistent, Reproducible Results
Using a controlled atmosphere removes the variability of ambient air. This ensures that every batch is processed under the exact same conditions, leading to highly reproducible and successful results, which is critical in any professional manufacturing environment.
Common Types of Annealing Atmospheres
The choice of atmosphere depends on the material being treated, the desired surface finish, and cost considerations.
Inert Gas Atmospheres
The simplest protective atmospheres consist of inert gases, which are non-reactive. Their sole purpose is to displace the oxygen in the furnace.
High-purity nitrogen (N₂) and argon (Ar) are the most common choices. They provide excellent protection against oxidation and are essential for materials like stainless steel and most non-ferrous metals to achieve a clean, bright finish.
Reactive Atmospheres
Some atmospheres are designed to be "active" or "reducing," meaning they can chemically react to remove light surface oxides that may already be present.
The most common example is endothermic gas, a mixture of nitrogen, carbon monoxide (CO), and hydrogen (H₂). The hydrogen and carbon monoxide content gives it reducing properties, making it highly effective for annealing steels.
Vacuum Atmospheres
A vacuum is the ultimate protective environment. By removing almost all gas molecules from the furnace chamber, a vacuum virtually eliminates the possibility of oxidation or other surface reactions.
Vacuum annealing is a high-purity process used for sensitive or reactive metals and when the absolute cleanest surface finish is required.
Understanding the Trade-offs
Selecting an atmosphere is a balance between technical requirements and operational costs. It is not always necessary to use the most complex or pure environment.
Cost vs. Required Outcome
Annealing in ambient air is the cheapest option, but it results in heavy scale formation. If this scale is acceptable or can be easily removed later, this may be a viable choice for low-cost carbon steels.
Conversely, using high-purity inert gases or operating a vacuum furnace involves significant equipment and operational costs. This expense is justified only when a scale-free, bright finish is a critical requirement for the part's function or appearance.
Complexity and Safety
Reactive atmospheres like endothermic gas require precise control systems to maintain the correct gas composition. An improperly controlled atmosphere can cause unwanted carburisation (adding carbon) instead of preventing decarburisation.
Furthermore, atmospheres containing hydrogen or carbon monoxide are flammable and toxic, respectively, requiring robust safety protocols and ventilation.
Making the Right Choice for Your Goal
The optimal atmosphere is determined by the material and the desired final condition of the component.
- If your primary focus is cost-effectiveness on common steels: Annealing in ambient air is an option, provided you have a secondary process planned for removing the resulting scale.
- If your primary focus is a clean, bright finish on stainless steel or non-ferrous metals: A high-purity inert gas atmosphere (like nitrogen or argon) or a vacuum is essential to prevent oxidation.
- If your primary focus is preventing surface decarburisation in high-carbon steels: A precisely controlled endothermic gas atmosphere is the standard industry approach for reliable results.
Ultimately, selecting the right annealing atmosphere is a critical decision that directly impacts the final quality, appearance, and performance of the metal component.
Summary Table:
| Goal / Material | Recommended Atmosphere | Key Benefit |
|---|---|---|
| Cost-effectiveness on common steels | Ambient Air (with scale removal) | Lowest cost |
| Clean, bright finish (stainless steel, non-ferrous) | Inert Gas (N₂, Ar) or Vacuum | Prevents oxidation |
| Prevent decarburisation (high-carbon steels) | Endothermic Gas | Maintains surface carbon content |
Achieve flawless annealing results with KINTEK.
Choosing the right atmosphere is critical for protecting your metal components from oxidation, scale, and decarburisation. KINTEK specializes in lab equipment and consumables, providing the precise furnace systems and gas control solutions your laboratory needs for consistent, high-quality annealing.
Our experts can help you select the ideal setup for your specific materials and quality requirements, ensuring you avoid costly rework and achieve perfect results every time.
Contact KINTEK today to discuss your annealing process and discover how we can enhance your lab's capabilities and efficiency.
Visual Guide
Related Products
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why controlled atmosphere furnace is desirable in sintering? Achieve Superior Purity and Density
- What role does a high-temperature hydrogen atmosphere furnace play in the heat treatment of tungsten plates?
- What is nitrogen annealing in metalworking? Enhancing Ductility and Preventing Oxidation for High-Quality Components
- What is the endothermic atmosphere? A Guide to Precision Steel Heat Treatment
- What is the role of nitrogen in annealing process? Creating a Controlled, Protective Atmosphere
- Why is a high-temperature atmosphere furnace necessary for nanocomposite catalysts? Master Atomic-Level Engineering
- What is the purpose of using a high-temperature heating furnace with atmosphere protection? Protect Your Composites
- What is an atmosphere oven? Achieve Precise Thermal Processing in a Controlled Gas Environment



















