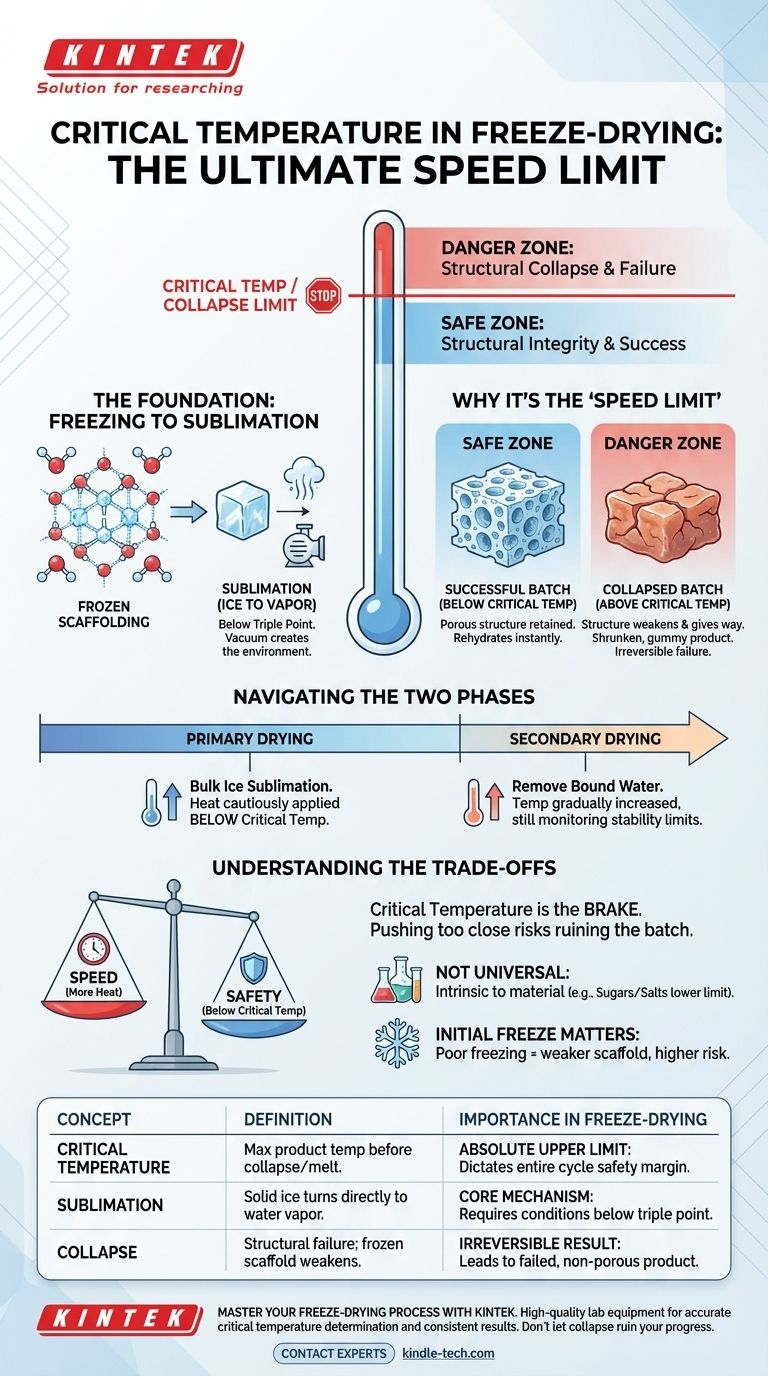
The critical temperature in freeze-drying is the absolute maximum temperature your product can withstand during the process before its solid structure collapses or it begins to melt. Exceeding this temperature leads to a failed batch, resulting in a gummy, shrunken product instead of a properly preserved one. This single value dictates the safety margin for the entire drying cycle.
The entire freeze-drying process is a balancing act between removing water and maintaining the product's frozen, solid structure. The critical temperature is the non-negotiable upper limit that dictates how aggressively you can apply heat to speed up drying without destroying the very structure you're trying to preserve.

The Foundation: From Freezing to Sublimation
The Goal: Turning Ice Directly to Vapor
Freeze-drying, or lyophilization, works by a process called sublimation. This is where a solid (ice) turns directly into a gas (water vapor), completely skipping the liquid phase.
This is only possible when the material is held below its "triple point"—the specific combination of low temperature and low pressure where solid, liquid, and gas can coexist.
Creating the Frozen Scaffolding
The initial freezing phase is the most critical step because it creates the product's foundational structure. The water within the product freezes into a network of ice crystals.
When this ice is later removed through sublimation, it leaves behind a porous, sponge-like structure. This structure is what allows the final product to be rehydrated instantly.
The Role of the Vacuum
After the product is frozen solid, a deep vacuum is applied to the chamber. This drastic reduction in pressure is what allows sublimation to occur at very low temperatures.
The vacuum effectively lowers the boiling point of water to below its freezing point, enabling the ice to "boil" away without ever melting.
Why Critical Temperature Is the "Speed Limit"
What Happens When You Exceed It: "Collapse"
The "critical temperature" is more accurately called the collapse temperature. The frozen ice crystals act as a rigid scaffold that holds up the product's structure.
If the product temperature rises too high, this scaffold weakens and can no longer support its own weight. The structure gives way in an event called collapse.
The result is a shrunken, dense, or sticky product that has lost its porous nature and will not rehydrate properly. It is an irreversible failure.
Navigating the Two Phases of Drying
Freeze-drying occurs in two main stages, and the critical temperature is the guiding principle for both.
Primary Drying is the longest phase, where heat is slowly introduced to provide the energy needed for the bulk of the ice to sublimate. The product temperature must be kept safely below the critical temperature throughout this stage.
Secondary Drying begins after all the ice crystals are gone. The temperature is then gradually increased to remove the final, tightly-bound water molecules from the product. Even here, exceeding the product's stability limits can cause damage.
Understanding the Trade-offs
The Tension Between Speed and Safety
Freeze-drying is an extremely slow, energy-intensive process. There is a constant temptation to add more heat to the shelves to speed up sublimation and shorten cycle times.
However, the critical temperature acts as the absolute brake on this desire. Pushing the temperature too close to this limit risks ruining the entire batch for the sake of a slightly shorter cycle.
Not a Universal Number
The critical temperature is not a single value for all products. It is an intrinsic property of the material being dried.
Products with high concentrations of sugars or salts, for example, have a much lower collapse temperature than products with more rigid cellular structures. Each unique formulation has its own specific limit.
The Impact of the Initial Freeze
A poor freezing protocol can lead to collapse even if you stay below the official critical temperature.
If the initial freeze is too slow, large and irregular ice crystals form, creating a weaker scaffold that is more susceptible to collapse during drying. A rapid, well-controlled freeze creates a stronger structure.
Making the Right Choice for Your Goal
To successfully apply this principle, you must align your process with your primary objective.
- If your primary focus is preserving fragile pharmaceuticals: You must operate well below the critical temperature to guarantee maximum structural integrity and product viability.
- If your primary focus is food preservation: You might operate closer to the critical temperature to optimize for faster cycle times, but you must monitor the product closely for any signs of collapse.
- If you are developing a new freeze-drying recipe: The first and most important step is to determine the specific critical temperature of your product, often using specialized lab equipment.
Understanding and respecting your product's critical temperature is the key to transforming this complex process into a reliable and repeatable success.
Summary Table:
| Concept | Definition | Importance in Freeze-Drying |
|---|---|---|
| Critical Temperature | Maximum product temperature before structural collapse/melting. | The absolute upper limit for heating; dictates the safety margin for the entire drying cycle. |
| Sublimation | Process where solid ice turns directly into water vapor. | The core mechanism of freeze-drying; requires temperatures below the product's triple point. |
| Collapse | Structural failure where the frozen scaffold weakens and gives way. | The irreversible result of exceeding the critical temperature; leads to a failed, non-porous product. |
| Primary Drying | The longest phase where the bulk of the ice sublimates. | Heat must be applied cautiously to keep the product temperature safely below its critical limit. |
Master Your Freeze-Drying Process with KINTEK
Understanding your product's unique critical temperature is the foundation of a successful lyophilization cycle. Whether you are preserving sensitive pharmaceuticals, optimizing food production, or developing a new formulation, having the right laboratory equipment is non-negotiable.
KINTEK specializes in supplying high-quality lab equipment and consumables tailored to your precise freeze-drying needs. We provide the reliable tools you need to accurately determine critical temperatures, monitor your process, and achieve consistent, high-quality results batch after batch.
Don't let a collapsed batch ruin your progress. Contact our experts today to discuss how KINTEK can support your laboratory's success with the right freeze-drying solutions.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- What role does a laboratory freeze dryer play in the synthesis of graphene-based electrocatalysts? Preserve 3D Structures
- Why is a freeze dryer preferred for reduced graphene oxide (Hh-RGO) powders? Preserve Nano-Structure and Performance
- Why is a freeze dryer preferred over thermal drying for Fe-ZTA cermets? Ensure Pure, Homogeneous Slurry Processing
- What are the advantages of using freeze drying for phase change materials with biopolymer shells? Optimize Stability
- What is the function of a freeze dryer in the ice-templating process? Preserving Aligned Pore Scaffolds for LAGP



















