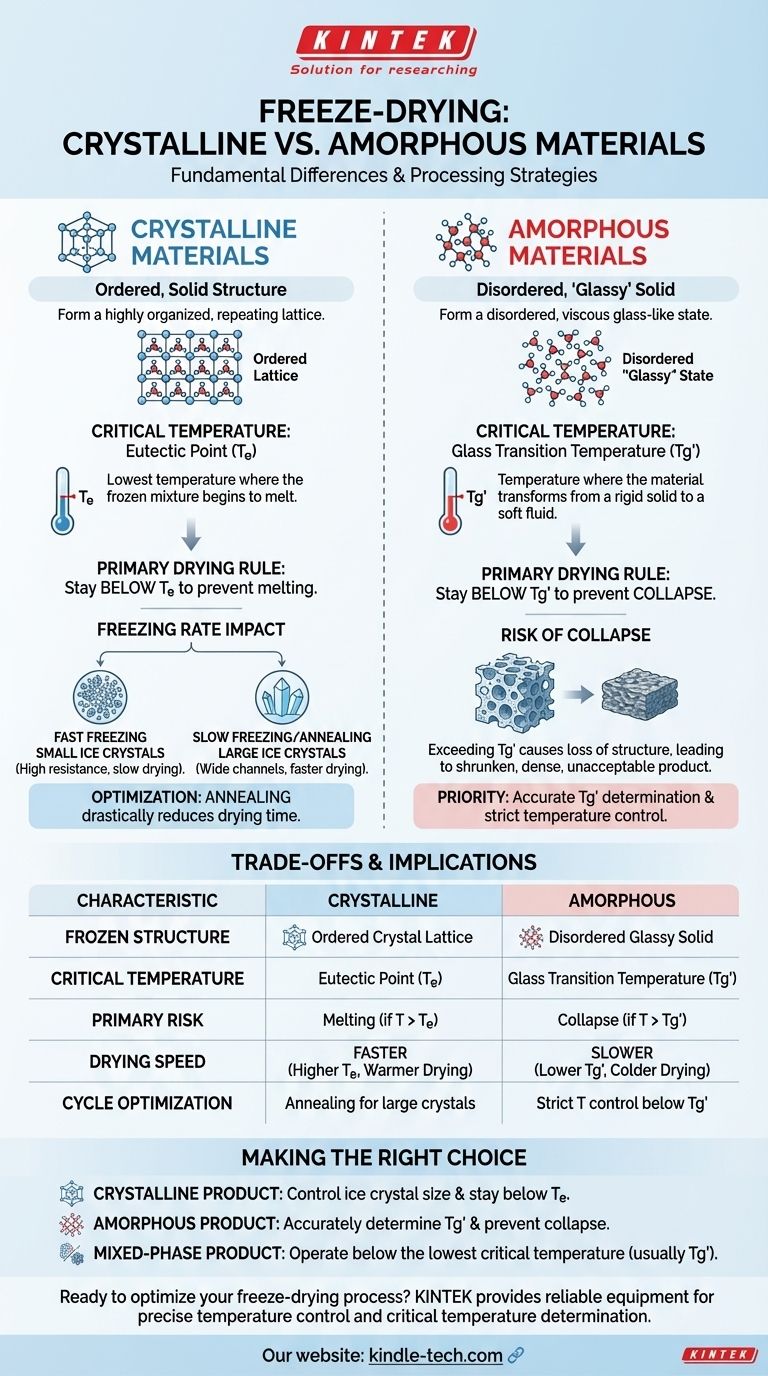
In freeze-drying, the fundamental difference is that crystalline materials form ordered, solid crystals with a distinct melting temperature, while amorphous materials form a disordered, "glassy" solid that softens over a range of temperatures. This structural difference dictates the entire strategy for successfully removing water without destroying the product.
The core distinction is the critical temperature you must stay below during drying. For crystalline materials, it is the eutectic point (
Te), where the product melts. For amorphous materials, it is the glass transition temperature (Tg'), where the product softens and collapses.

Understanding the Crystalline State
The behavior of crystalline materials in freeze-drying is governed by the formation of a predictable, ordered structure.
What Defines a Crystal?
When frozen, these materials arrange their molecules into a highly organized, repeating lattice. This structure is rigid and stable.
The frozen matrix consists of pure ice crystals separated from crystals of the solute (the substance being freeze-dried).
The Eutectic Point (Te): The Critical Threshold
A crystalline mixture doesn't have a single melting point but a eutectic temperature (Te). This is the lowest possible temperature at which the frozen mixture can begin to melt.
To prevent the product from liquefying, the primary drying phase of freeze-drying must be performed at a temperature below the eutectic point.
The Impact of Freezing Rate
The speed of freezing directly impacts the size of the ice crystals that form.
Fast freezing creates many small ice crystals. These are difficult to dry because they create a dense network with high resistance to water vapor flow.
Slow freezing or annealing (holding the product at a temperature just below Te) allows for the formation of larger, more uniform ice crystals. This creates wider channels for vapor to escape, significantly speeding up the drying process.
Understanding the Amorphous State
Amorphous materials, often complex multi-component mixtures, behave very differently because they never form an ordered crystal structure.
What Is an Amorphous "Glass"?
Upon freezing, these materials do not crystallize. Instead, the water freezes into ice crystals, and the remaining solutes become so concentrated and viscous that they solidify into a disordered, glass-like state.
This glassy phase is what provides the structural support for the product once the ice is removed.
The Glass Transition Temperature (Tg'): The Critical Threshold
Amorphous materials do not have a eutectic point. Instead, they have a glass transition temperature (Tg').
Below the Tg', the material is a rigid, brittle solid. Above the Tg', it transforms into a soft, rubbery, and viscous fluid.
During freeze-drying, if the product temperature exceeds Tg', the glassy structure will soften and lose its ability to support itself, leading to product collapse. Therefore, primary drying must occur below this temperature.
Understanding the Trade-offs and Implications
The state of your material—crystalline or amorphous—directly dictates your processing strategy, efficiency, and potential points of failure.
Critical Temperature Determines Drying Speed
Crystalline materials often have a higher eutectic temperature compared to the glass transition temperature of many amorphous products.
A higher critical temperature allows you to run the primary drying phase warmer and at a lower vacuum, which significantly shortens the overall cycle time. Amorphous products with low Tg' values require colder, longer, and more expensive drying cycles.
The Risk of Collapse in Amorphous Products
Collapse is the primary failure mode for amorphous materials. Exceeding the Tg' causes the solid matrix to flow, destroying the porous structure needed for sublimation and resulting in a shrunken, dense, and unacceptable final product.
The Advantage of Annealing for Crystalline Products
Annealing is a powerful tool for crystalline formulations. By promoting the growth of large ice crystals, you can drastically reduce the time required for primary drying. This technique is generally specific to optimizing crystalline systems.
Making the Right Choice for Your Goal
Your approach to developing a freeze-drying cycle depends entirely on the physical nature of your frozen product.
- If you are working with a crystalline product: Your focus should be on controlling ice crystal size through the freezing rate and potential annealing steps, while ensuring the product temperature remains below the eutectic point (
Te). - If you are working with an amorphous product: Your absolute priority is to accurately determine the glass transition temperature (
Tg') and design a drying cycle that keeps the product safely below it to prevent structural collapse. - If you have a mixed-phase product (partially crystalline): You must identify and operate below the lowest critical temperature of the system, which is almost always the
Tg'of the amorphous portion.
Ultimately, knowing whether your material is crystalline or amorphous is the foundational step in designing a robust, efficient, and successful freeze-drying process.
Summary Table:
| Characteristic | Crystalline Materials | Amorphous Materials |
|---|---|---|
| Frozen Structure | Ordered, rigid crystal lattice | Disordered, glassy solid |
| Critical Temperature | Eutectic Point (Te) | Glass Transition Temperature (Tg') |
| Primary Risk | Melting (if T > Te) | Collapse (if T > Tg') |
| Cycle Optimization | Annealing for larger ice crystals | Strict temperature control below Tg' |
| Typical Drying Speed | Faster (higher Te allows warmer drying) | Slower (lower Tg' requires colder drying) |
Ready to optimize your freeze-drying process?
The right lab equipment is critical for maintaining precise temperatures and preventing product failure. KINTEK specializes in providing reliable freeze dryers and thermal analysis tools that help you accurately determine critical temperatures like Te and Tg' for both crystalline and amorphous materials.
We serve laboratories in pharmaceuticals, biotechnology, and food science, ensuring your products are dried efficiently and effectively. Contact our experts today to discuss your specific application and find the perfect solution for your lab's needs.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- Why is a freeze dryer preferred for reduced graphene oxide (Hh-RGO) powders? Preserve Nano-Structure and Performance
- Why is a freeze dryer preferred for drying nickel nanoparticle precursors? Prevent Hard Agglomeration Now
- Why is a laboratory vacuum freeze dryer essential for plant extracts? Preserve Bioactivity & Structure
- What is the function of a freeze dryer in the ice-templating process? Preserving Aligned Pore Scaffolds for LAGP
- What are the advantages of using freeze drying for phase change materials with biopolymer shells? Optimize Stability



















