In short, catalysts are used in pyrolysis to selectively control the chemical reactions, lowering the process temperature while improving the quality and yield of desired products like biofuels and valuable chemicals. Instead of just breaking down material with heat, a catalyst actively guides the formation of specific molecules, transforming the process from simple decomposition into a more precise form of chemical synthesis.
Catalysts are not merely accelerators for pyrolysis; they are steering agents. Their primary effect is to provide a controlled environment that favors specific reaction pathways, allowing for the targeted production of higher-value outputs from a given feedstock.

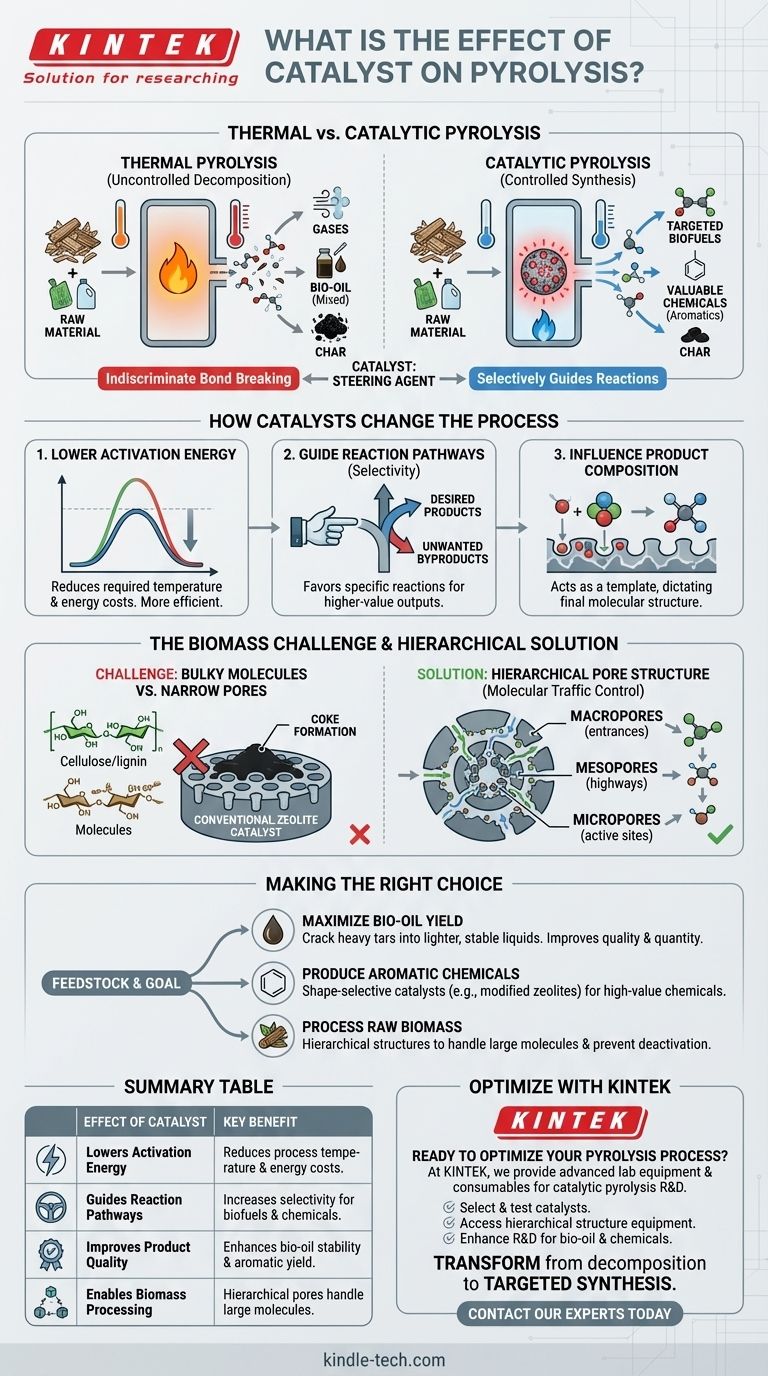
How Catalysts Fundamentally Change Pyrolysis
Pyrolysis without a catalyst is driven purely by thermal energy, which indiscriminately breaks chemical bonds. Adding a catalyst introduces a new layer of control over the entire process.
Lowering the Activation Energy
The most fundamental role of a catalyst is to lower the energy required to initiate chemical reactions. This means pyrolysis can occur at lower temperatures, reducing energy consumption and operational costs.
Guiding Reaction Pathways
A catalyst provides an active surface where reactant molecules can adsorb, react, and desorb as new products. By design, this surface favors certain reactions over others, a property known as selectivity.
This allows you to steer the decomposition of large, complex molecules toward a specific, more valuable output, such as aromatic hydrocarbons, instead of a random mixture of gases, liquids (bio-oil), and char.
Directly Influencing Product Composition
The catalyst is not a passive bystander; it actively participates in the reaction. For example, in some processes, atoms from the feedstock can dissolve into the catalyst's structure.
This interaction directly influences the final properties of the product. The catalyst's chemical and physical structure dictates what molecules can form, effectively acting as a template for the desired output.
The Challenge of Catalysts in Biomass Pyrolysis
While catalytic pyrolysis is powerful, applying it to biomass presents unique challenges that standard industrial catalysts were not designed to handle.
The Problem of Molecular Bulk
Biomass is composed of large natural polymers like cellulose and lignin. These molecules are significantly bulkier than the smaller petrochemical molecules for which many commercial catalysts were originally developed.
Limitations of Conventional Catalysts
Many common commercial catalysts, such as zeolite-based catalysts, are microporous. They contain extremely narrow pores and channels where the chemical reactions take place.
These narrow pores create a significant barrier for bulky biomass molecules. The large molecules cannot enter the catalyst's internal structure to reach the active sites, rendering the catalyst ineffective. This often leads to coke formation on the catalyst's exterior, causing rapid deactivation.
Overcoming Challenges with Advanced Catalyst Design
To solve the incompatibility between large biomass molecules and small catalyst pores, researchers focus on engineering the catalyst's physical structure.
Creating a Hierarchical Pore Structure
The most effective solution is to design catalysts with a multidimensional or hierarchical structure. This involves creating different sizes of pores within a single catalyst particle:
- Macropores (large): Act as the main entrance, allowing large molecules to get inside.
- Mesopores (medium): Serve as highways to transport molecules deeper into the catalyst.
- Micropores (small): Contain the active sites where the final, precise chemical conversions occur.
Improving "Molecular Traffic Control"
This hierarchical structure creates excellent molecular traffic control. Large biomass-derived molecules can easily access the catalyst's interior through the larger pores, where they are broken down into smaller intermediates.
These smaller molecules can then enter the narrow micropores to be converted into the final, desired products. This dramatically improves efficiency and extends the catalyst's lifespan by preventing pore blockage.
Making the Right Choice for Your Goal
The choice of a catalytic strategy depends entirely on your feedstock and desired output.
- If your primary focus is maximizing liquid bio-oil yield: A catalyst's primary role is to crack heavy, low-value tars into lighter, more stable liquid compounds, improving the overall quality and quantity of the oil.
- If your primary focus is producing specific aromatic chemicals: Shape-selective catalysts like modified zeolites are necessary to control the final molecular structure and produce high-value chemicals.
- If your primary focus is processing raw biomass feedstock: You must prioritize catalysts with a hierarchical pore structure to handle the large molecules efficiently and avoid rapid deactivation.
By carefully selecting your catalyst, you transform pyrolysis from a crude decomposition process into a sophisticated tool for chemical manufacturing.
Summary Table:
| Effect of Catalyst | Key Benefit |
|---|---|
| Lowers Activation Energy | Reduces required process temperature and energy costs. |
| Guides Reaction Pathways | Increases selectivity for desired products (e.g., biofuels, chemicals). |
| Improves Product Quality | Enhances bio-oil stability and yield of valuable aromatics. |
| Enables Biomass Processing | Hierarchical pore structures handle large feedstock molecules. |
Ready to optimize your pyrolysis process with the right catalyst?
At KINTEK, we specialize in providing advanced laboratory equipment and consumables tailored for catalytic pyrolysis research and development. Whether you are working with biomass, plastics, or other feedstocks, our solutions help you achieve precise control over reaction pathways, improve product yields, and scale your process efficiently.
We help you:
- Select and test catalysts for specific feedstocks and desired outputs.
- Access equipment designed for hierarchical catalyst structures and molecular traffic control.
- Enhance your R&D with reliable tools for bio-oil upgrading and chemical production.
Transform your pyrolysis from simple decomposition to targeted synthesis. Contact our experts today to discuss your catalytic pyrolysis needs and how KINTEK can support your innovation.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Custom PTFE Wafer Holders for Lab and Semiconductor Processing
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
People Also Ask
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time



















