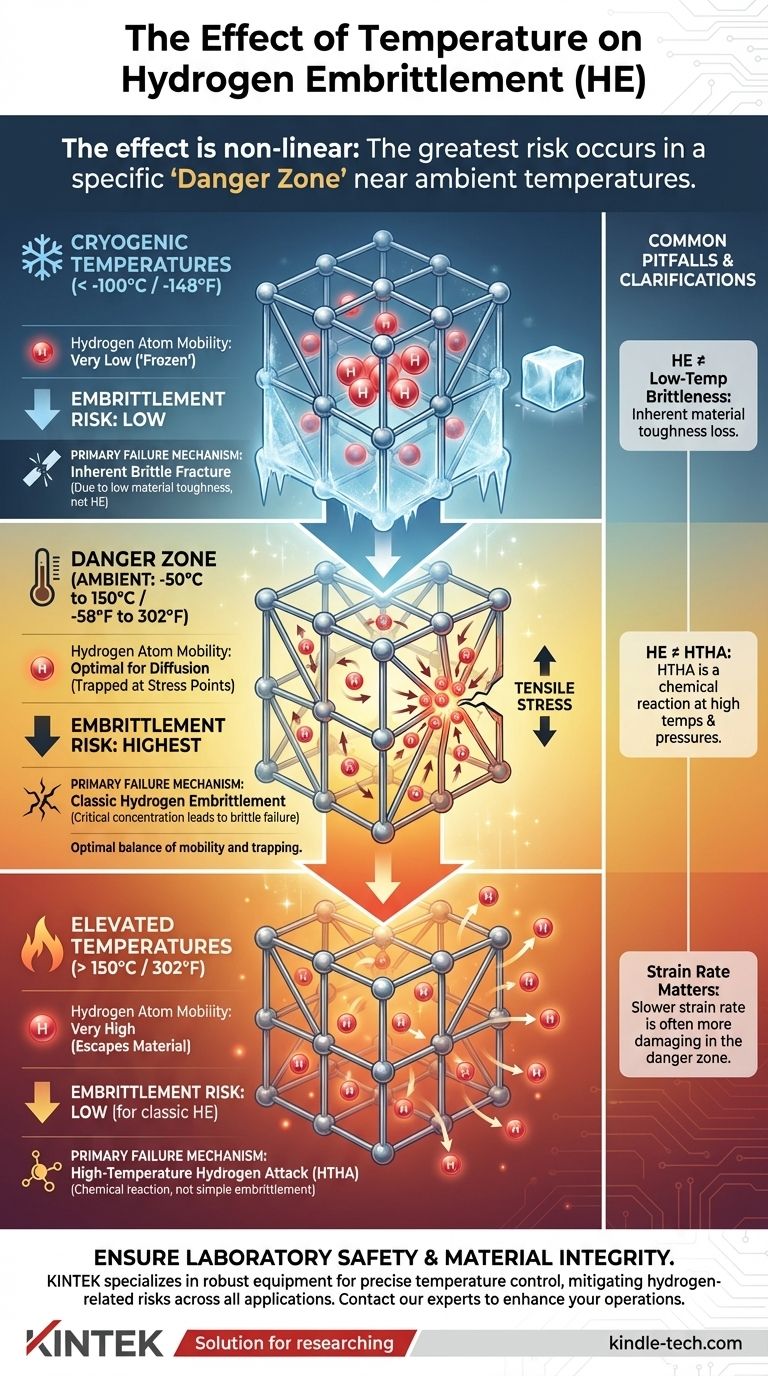
In short, the effect of temperature on hydrogen embrittlement is not linear. The phenomenon is most severe in a specific range around room temperature. Both very low (cryogenic) and elevated temperatures significantly reduce the risk of classic hydrogen embrittlement, but for entirely different reasons related to hydrogen atom mobility.
The greatest risk of failure from hydrogen embrittlement occurs near ambient temperatures, from approximately -50°C to 150°C (-58°F to 302°F). This temperature window creates a dangerous balance where hydrogen atoms are mobile enough to find stress points but not so energetic that they escape the material.

The Underlying Mechanics: Hydrogen Mobility
To understand the role of temperature, we must first recognize that hydrogen embrittlement requires three conditions: a susceptible material (like high-strength steel), applied tensile stress, and a source of atomic hydrogen. Temperature's primary role is to govern the behavior of these hydrogen atoms within the metal's crystal lattice.
H3: The "Danger Zone" (Near-Ambient Temperatures)
This range represents the highest risk because it provides the optimal conditions for embrittlement.
Hydrogen atoms have enough thermal energy to diffuse, or move, through the metal. This mobility allows them to migrate and accumulate in areas of high stress, such as the tip of a microscopic crack.
Simultaneously, the temperature is not high enough for the hydrogen to easily diffuse back out of the material. This combination of sufficient mobility and effective trapping leads to a critical concentration of hydrogen at stress points, severely reducing the material's ductility and leading to sudden, brittle failure.
H3: Low Temperatures (Cryogenic Conditions)
As temperature drops significantly (e.g., below -100°C / -148°F), the risk of classic hydrogen embrittlement decreases.
At these cryogenic temperatures, the diffusion rate of hydrogen atoms becomes extremely slow. The atoms are essentially "frozen" in place within the metal lattice.
Because they lack the mobility to travel to areas of high tensile stress, they cannot accumulate to the critical concentrations needed to cause embrittlement.
H3: Elevated Temperatures
At higher temperatures (e.g., above 150°C / 302°F), the risk of classic hydrogen embrittlement also falls off, but for the opposite reason.
The diffusion rate of hydrogen becomes very high. This extreme mobility means hydrogen atoms can easily diffuse out of the material into the atmosphere, preventing dangerous internal accumulations.
Additionally, at these temperatures, the metal itself becomes more ductile and its yield strength decreases, making it inherently less prone to brittle fracture.
Common Pitfalls and Clarifications
A clear understanding of the temperature effect requires differentiating hydrogen embrittlement from other temperature-dependent failure mechanisms.
H3: Do Not Confuse HE with Low-Temperature Brittleness
While HE risk is low at cryogenic temperatures, the risk of a different failure mode—brittle fracture—is very high for many steels. This is due to the inherent loss of toughness in the material itself at low temperatures and is a separate phenomenon.
H3: Distinguish HE from High-Temperature Hydrogen Attack (HTHA)
At very high temperatures (typically above 200°C / 400°F) and in high-pressure hydrogen gas environments, a different mechanism called High-Temperature Hydrogen Attack (HTHA) can occur.
This is not a simple embrittlement process but a chemical reaction. Hydrogen reacts with carbides in the steel to form methane gas, leading to internal cracking, blistering, and a permanent loss of strength. HTHA is a fundamentally different and irreversible form of material degradation.
H3: Consider the Impact of Strain Rate
The embrittlement process is time-dependent. In the "danger zone," a slower strain rate is often more damaging because it gives hydrogen atoms more time to diffuse to the tip of a propagating crack, exacerbating the problem.
Making the Right Choice for Your Application
Your approach to mitigating hydrogen-related failures must be tailored to the specific operating temperature range of your component.
- If your primary focus is cryogenic service (below -100°C): Your main concern is the material's intrinsic toughness, not classic hydrogen embrittlement. Select materials with excellent Charpy V-notch impact values at your minimum design temperature.
- If your component operates near ambient temperatures (-50°C to 150°C): You are in the highest risk zone. Prioritize selecting less susceptible materials, rigorously controlling all potential hydrogen sources (e.g., manufacturing processes like plating, welding, or in-service corrosion), and carefully managing tensile stresses.
- If you are operating at elevated temperatures (above 150°C): The risk of classic embrittlement is lower, but you must shift your analysis to the separate and severe risk of High-Temperature Hydrogen Attack (HTHA), especially for carbon and low-alloy steels in hydrogen service.
Ultimately, temperature is the critical variable that dictates whether hydrogen within a material is a benign passenger or a catalyst for catastrophic failure.
Summary Table:
| Temperature Range | Hydrogen Atom Mobility | Embrittlement Risk | Primary Failure Mechanism |
|---|---|---|---|
| Cryogenic (< -100°C / -148°F) | Very Low ("Frozen") | Low | Inherent Brittle Fracture (Material Toughness) |
| Danger Zone (-50°C to 150°C / -58°F to 302°F) | Optimal for Diffusion | Highest | Classic Hydrogen Embrittlement |
| Elevated (> 150°C / 302°F) | Very High (Escapes Material) | Low (for HE) | High-Temperature Hydrogen Attack (HTHA) |
Ensure your laboratory materials and equipment are safe from hydrogen-related failures. KINTEK specializes in providing robust lab equipment and consumables designed for precise temperature control and material integrity. Whether you are working with cryogenic applications, ambient conditions, or high-temperature processes, our solutions help mitigate the risks of hydrogen embrittlement and other failure mechanisms. Contact our experts today to discuss how we can support your laboratory's specific needs and enhance the safety and reliability of your operations.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Electric Split Lab Cold Isostatic Press CIP Machine for Cold Isostatic Pressing
People Also Ask
- What is the role of high-pressure reactors in the study of alloy oxidation? Essential Tools for Supercritical Research
- What is the purpose of using a high-temperature hydrothermal reactor? Enhance Iodine@Activated Carbon Cathode Synthesis
- Why are high-pressure autoclaves essential for preparing bio-based polyamide curing agents from dimeric acid?
- Why are sealed laboratory reaction vessels necessary in the hydrothermal synthesis of zeolites? Ensure Purity and Yield
- What is the function of a constant temperature hydrothermal reactor? Master Coal Fly Ash Activation



















