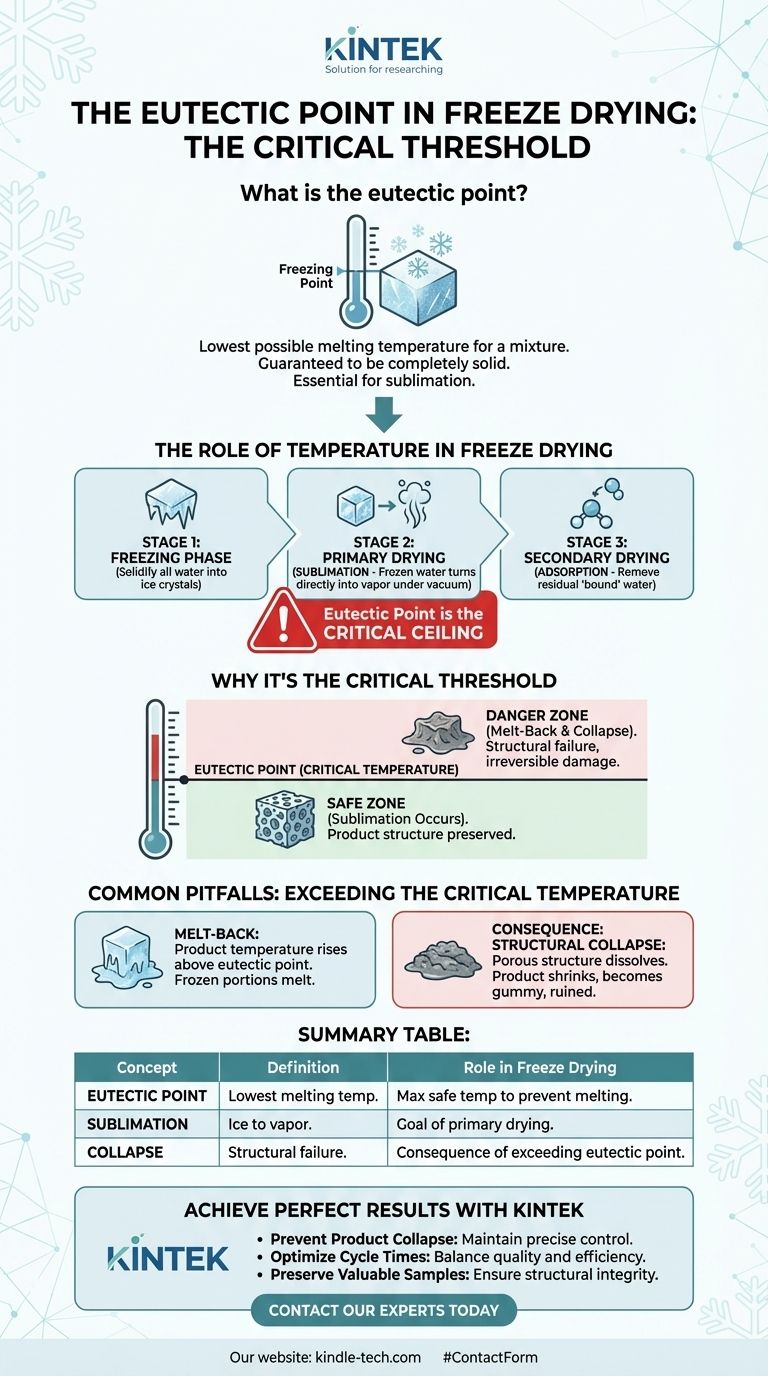
In freeze drying, the eutectic point is the lowest possible melting temperature for a mixture of substances. It represents the critical threshold where the product is guaranteed to be completely solid, which is the foundational requirement for turning its frozen water content directly into vapor.
The core challenge in freeze drying is removing water without destroying the product's physical structure. The eutectic point serves as a critical temperature ceiling during the drying phase—staying below it prevents the product from melting, which would cause it to collapse and ruin the final result.

The Role of Temperature in the Freeze-Drying Process
Freeze drying, or lyophilization, is a precise, three-stage process governed by temperature and pressure. Understanding these stages reveals why a single temperature point can be so important.
Stage 1: The Freezing Phase
The first and most essential step is to completely freeze the material. The goal is to solidify all the water within the product into ice crystals. An incomplete freeze will lead to failure in the subsequent stages.
Stage 2: Primary Drying (Sublimation)
This is where the magic happens. The pressure in the chamber is lowered to create a deep vacuum, and a small amount of heat is applied. This combination allows the frozen water to bypass the liquid phase and turn directly into a vapor, a process called sublimation.
This stage is the longest and removes about 95% of the water. The cold condenser in the freeze dryer collects this water vapor, turning it back into ice away from the product.
Stage 3: Secondary Drying (Adsorption)
After all the free ice has been sublimated, a small amount of "bound" water molecules remains attached to the product itself. In this final phase, the temperature is raised slightly and the vacuum is often increased to remove this residual moisture.
Why the Eutectic Point is the Critical Threshold
The success of the entire primary drying stage hinges on keeping the product temperature below a specific limit.
Defining the Critical Temperature
Every product has a critical temperature, which is the absolute maximum temperature it can withstand during freeze drying before its physical structure is compromised.
Exceeding this temperature causes the delicate, porous structure created by the ice crystals to break down, a failure known as collapse.
The Eutectic Point as the Specific Limit
For products that form a crystalline structure when frozen, the eutectic point is this critical temperature. It is the precise point on the thermometer where the solid matrix begins to melt.
Therefore, to ensure the ice sublimates rather than melts, the product temperature must be kept safely below its eutectic point throughout the entire primary drying phase. It's important to note that not all products have a single, clear eutectic point.
Common Pitfalls: Exceeding the Critical Temperature
Ignoring the eutectic point is the most common cause of failed freeze-drying cycles. This oversight leads to irreversible product damage.
The Problem of "Melt-Back"
If the heat added during primary drying raises the product's temperature above its eutectic point, the frozen portions will simply melt. This liquid water cannot be removed by sublimation.
The Consequence: Structural Collapse
When melting occurs, the solid scaffolding that holds the product's shape dissolves. The product loses its porous structure, shrinks, and often turns into a gummy or shriveled mass.
This not only ruins the appearance but also degrades biological activity, flavor, and the ability to be easily reconstituted. The primary benefits of freeze drying are lost entirely.
Making the Right Choice for Your Goal
Controlling the process temperature relative to the eutectic point is fundamental to a successful outcome.
- If your primary focus is maximum product quality: You must identify your product's eutectic point and ensure your shelf temperature during primary drying keeps the product safely below this threshold.
- If your primary focus is process efficiency: Carefully test how close you can bring the product temperature to the eutectic point to speed up sublimation without risking melt-back.
Ultimately, understanding and respecting your product's critical temperature is the key principle that separates successful freeze drying from costly failure.
Summary Table:
| Concept | Definition | Role in Freeze Drying |
|---|---|---|
| Eutectic Point | The lowest melting temperature of a mixture. | The maximum safe temperature during primary drying to prevent melting. |
| Sublimation | The process where ice turns directly into vapor. | The goal of primary drying; only possible if product is fully frozen. |
| Collapse | The structural failure of a product due to melting. | The consequence of exceeding the eutectic point, ruining the product. |
Achieve Perfect Freeze-Drying Results with KINTEK
Understanding the critical role of the eutectic point is the first step. The next is having the right equipment to precisely control it. KINTEK specializes in high-performance laboratory freeze dryers designed for unparalleled temperature accuracy and process reliability.
Our solutions help you:
- Prevent Product Collapse: Maintain precise control to stay safely below your product's critical temperature.
- Optimize Cycle Times: Balance quality and efficiency with reliable, consistent performance.
- Preserve Valuable Samples: Ensure the structural integrity and biological activity of pharmaceuticals, food, and research materials.
Don't let an uncontrolled temperature ruin a valuable batch. Contact our lyophilization experts today to find the perfect freeze-drying solution for your laboratory's specific needs.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- What is the function of a freeze dryer in the ice-templating process? Preserving Aligned Pore Scaffolds for LAGP
- What is the function of Freeze-thaw Equipment in Au-(PNiPAAm/PVA) hydrogel? Achieve High-Speed Photothermal Actuation
- What role does a laboratory freeze dryer play in the synthesis of graphene-based electrocatalysts? Preserve 3D Structures
- Why is a freeze dryer preferred for drying nickel nanoparticle precursors? Prevent Hard Agglomeration Now
- Why is a freeze dryer preferred for reduced graphene oxide (Hh-RGO) powders? Preserve Nano-Structure and Performance



















