At its core, a calciner is a specialized industrial furnace designed to heat solid materials to a high, precisely controlled temperature without melting them. Its primary function is to induce a chemical reaction or a physical phase change in the material. This process, known as calcination, is used to remove volatile substances like water and carbon dioxide, or to change the material's crystal structure to give it new, desired properties.
A calciner is not merely an oven; it is a thermal reactor. Its purpose is to transform a raw material into an entirely new substance with specific chemical and physical characteristics by meticulously managing heat, time, and atmospheric conditions.

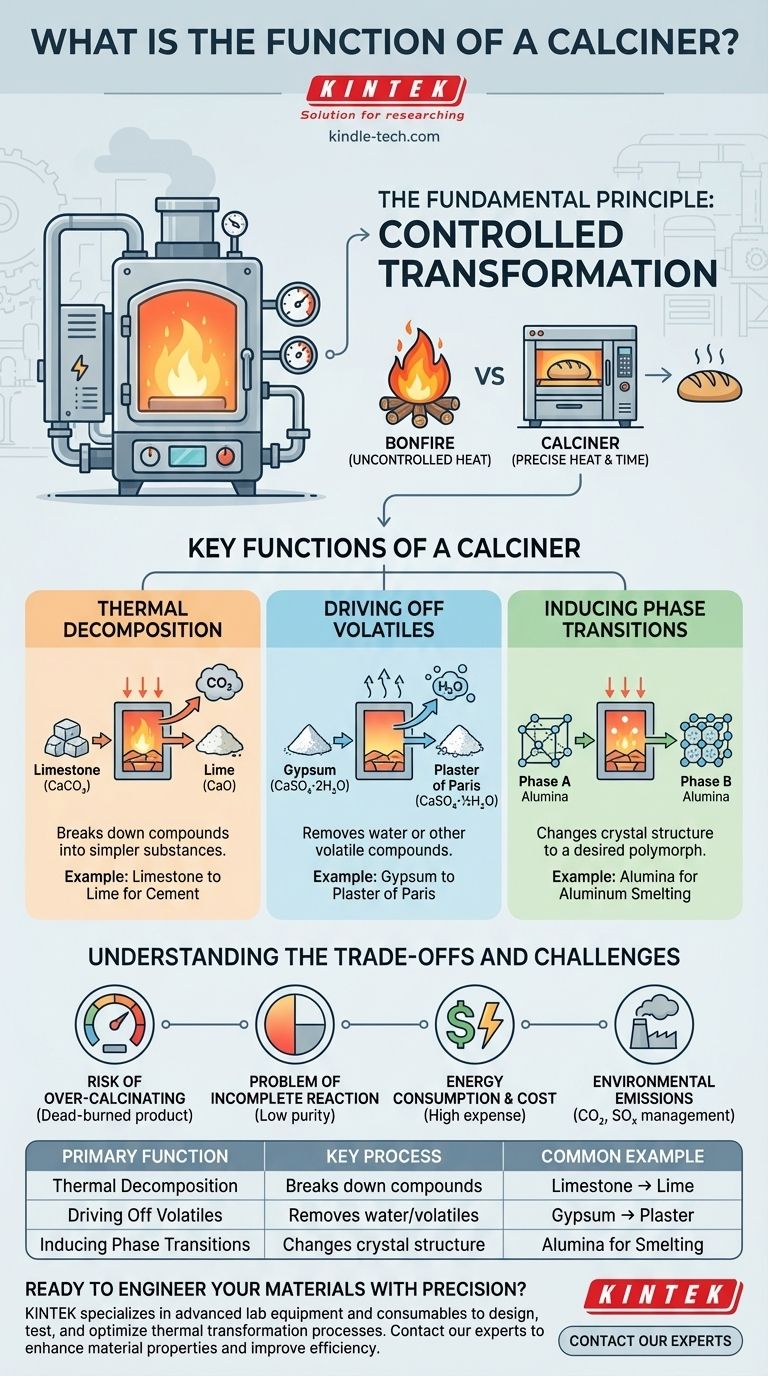
The Fundamental Principle: Controlled Transformation
Think of a calciner not as a bonfire, but as a sophisticated baker's oven. A bonfire applies uncontrolled heat, whereas a baker's oven applies precise heat for a specific time to transform dough into bread. The calciner operates on this same principle of controlled transformation.
Beyond Simple Heating
The goal of calcination is not just to make something hot. It is to use thermal energy as a tool to break chemical bonds or reorganize a material's internal structure. This requires precise control over the temperature profile—how quickly the material heats up, how long it stays at the peak temperature, and how it cools.
The Goal: A New Material
The material that exits a calciner is fundamentally different from the one that entered. It may be lighter, more porous, more chemically reactive, or have a different crystalline form. The entire process is engineered to produce this specific output reliably and consistently.
Key Functions of a Calciner
A calciner performs several distinct functions, often simultaneously, depending on the material and the desired outcome.
Thermal Decomposition
This is the classic function of a calciner. It involves heating a compound until it breaks down into two or more simpler substances.
The most common example is the production of lime (calcium oxide) from limestone (calcium carbonate) for the cement industry. When heated to over 900°C (1650°F), the limestone decomposes, releasing carbon dioxide gas and leaving behind the highly reactive lime.
Driving Off Volatiles
This function involves removing water or other volatile compounds from a material. This can include removing simple moisture or, more importantly, chemically bound water known as "water of hydration."
A key example is processing gypsum to make plaster of Paris. The calciner carefully heats the gypsum to drive off a specific amount of its water molecules, changing its chemical structure and allowing it to be rehydrated later to form a hard solid.
Inducing Phase Transitions
Some materials can exist in different solid forms, or crystal structures, known as polymorphs. A calciner can be used to heat a material to a specific temperature to force it to convert from one phase to another.
This is critical in the production of alumina, the precursor to aluminum. Different phases of alumina have different properties, and calcination is used to produce the specific phase required for the smelting process.
Understanding the Trade-offs and Challenges
Operating a calciner involves a delicate balance. Mismanaging the process can lead to significant problems, impacting product quality and operational efficiency.
The Risk of Over-Calcining
Heating a material for too long or at too high a temperature can create an undesirable product. For example, "dead-burned" lime is created when it is over-calcined, making it dense and chemically unreactive, which is useless for many applications like cement production.
The Problem of Incomplete Reaction
Conversely, not heating the material enough or for a sufficient duration results in an incomplete transformation. This leaves unreacted raw material in the final product, reducing its purity and performance.
Energy Consumption and Cost
Calciners operate at extremely high temperatures and are often massive pieces of equipment. As a result, they are incredibly energy-intensive. Fuel or electricity costs represent a major portion of the operational expense, making energy efficiency a critical design and operational concern.
Environmental Emissions
The very purpose of calcination is often to drive off gases like CO2 (from limestone) or SOx (from certain ores). These emissions must be captured, treated, or otherwise managed to comply with environmental regulations, adding complexity and cost to the operation.
How to Think About Calcination for Your Goal
The specific design and operation of a calciner system are dictated entirely by the desired properties of the final product.
- If your primary focus is creating a highly reactive product (like lime for cement): Your priority must be precise temperature control to achieve full conversion without causing dead-burning.
- If your primary focus is material purity and a specific crystal structure (like for catalysts or technical ceramics): You must prioritize meticulous control over both the temperature profile and the kiln atmosphere (e.g., oxygen-rich vs. oxygen-poor).
- If your primary focus is simple bulk drying or moisture removal: A less complex, lower-temperature system focused on maximizing residence time and airflow may be the most efficient solution.
Ultimately, a calciner is an essential industrial tool for turning a raw, common substance into a highly engineered, value-added material.
Summary Table:
| Primary Function | Key Process | Common Example |
|---|---|---|
| Thermal Decomposition | Breaks down compounds into simpler substances | Limestone to Lime (CaCO₃ → CaO + CO₂) |
| Driving Off Volatiles | Removes water or other volatile compounds | Gypsum to Plaster of Paris |
| Inducing Phase Transitions | Changes crystal structure to a desired polymorph | Alumina production for aluminum smelting |
Ready to Engineer Your Materials with Precision?
Whether you need to develop a highly reactive product, achieve specific crystal structures for technical ceramics, or efficiently remove volatiles, the right calcination process is critical. KINTEK specializes in providing advanced lab equipment and consumables to help you design, test, and optimize your thermal transformation processes.
Contact our experts today to discuss how our solutions can enhance your material properties, improve efficiency, and drive innovation in your laboratory or pilot plant.
Visual Guide
Related Products
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why is a vacuum drying oven necessary for treating WTaVTiZrx powder? Achieve High-Density, Defect-Free Laser Cladding
- What are the advantages of a vacuum drying oven for nZVI? Preserve Chemical Reactivity & Prevent Oxidation
- Are steel containing carbon used for carburizing? The Right Steel for a Hard Surface & Tough Core
- How does a sintering furnace influence EDC powder metallurgy electrodes? Optimize Your Tool for Superior Coatings
- What is the pressure range for pyrolysis? Optimize Product Yields with Precise Control
- What is the process of sintering a furnace? Achieve Precise Material Densification and Lining Durability
- What products are annealed? A Guide to Metals Requiring a Thermal 'Reset'
- What role does a vacuum resistance furnace play in the diffusion chromizing of steel? Achieve 2.8mm Deep Bonding



















