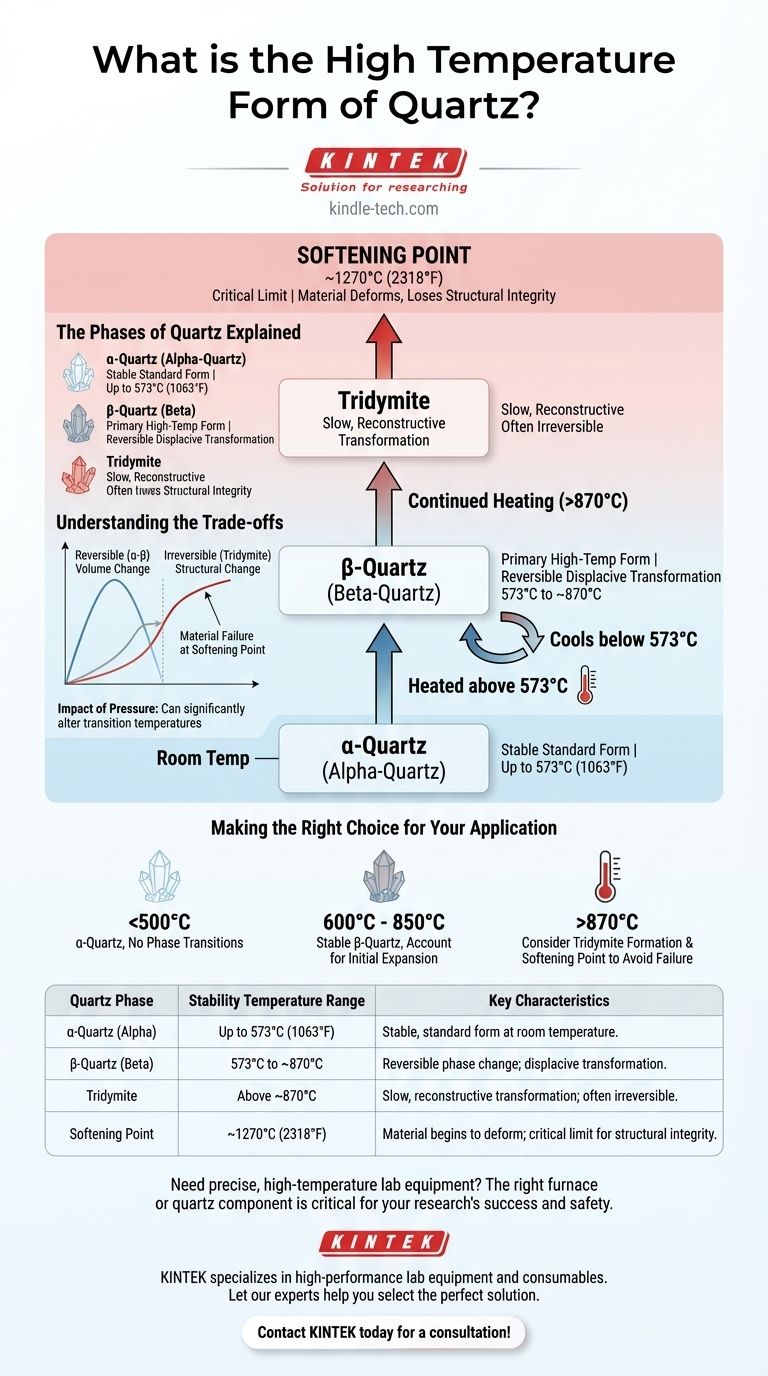
At atmospheric pressure, the primary high-temperature form of quartz is known as β-quartz (beta-quartz). This transformation from standard α-quartz (alpha-quartz) occurs spontaneously and reversibly once the material is heated above approximately 573°C (1063°F). However, this is only the first of several changes quartz undergoes at elevated temperatures.
Understanding quartz at high temperatures requires looking beyond a single transformation. The material undergoes a sequence of phase changes—from α-quartz to β-quartz, and then to other forms like tridymite—before reaching its practical limit, the softening point where it loses structural integrity.

The Phases of Quartz Explained
To use quartz effectively in any high-temperature setting, it's essential to understand its distinct thermal phases. These are not failures, but predictable changes in the material's crystal structure.
α-Quartz (Alpha-Quartz): The Standard Form
α-quartz is the crystalline form of silicon dioxide that is stable at room temperature and up to 573°C. This is the quartz found in nature and used in most standard applications, from electronics to countertops.
The Transition to β-Quartz (Beta-Quartz)
At approximately 573°C, α-quartz instantly reorganizes its crystal lattice to become β-quartz. This change is known as a displacive transformation; it's a subtle shift in atomic positions, not a complete rebuilding of the structure.
Critically, this process is reversible. As the material cools back below 573°C, it will immediately revert from β-quartz to α-quartz.
Tridymite: The Next Transformation
If you continue to heat the material, another change occurs. Above approximately 870°C, β-quartz will slowly begin to transform into tridymite, another crystalline polymorph of silicon dioxide.
Unlike the rapid α-β transition, this change is reconstructive, meaning atomic bonds are broken and reformed. It is a much slower and less easily reversed process.
Understanding the Trade-offs: From Phase Change to Material Failure
Knowing the phase transition temperatures is theoretical. For practical applications, you must also understand the material's physical limitations and how these changes impact its use.
Reversible vs. Irreversible Changes
The α-β transition at 573°C causes a small but immediate change in volume. Repeatedly cycling across this temperature can induce mechanical stress and micro-fractures in the material over time.
The transition to tridymite is much more significant and generally considered a permanent structural change in most practical scenarios.
Structural Integrity vs. Crystalline Form
The most critical limitation is not a phase change, but the softening point. Quartz glass begins to lose its rigidity and deform at around 1270°C (2318°F).
This is a hard limit for any application where structural integrity is required, such as in furnace tubes or reaction vessels. For instance, continuous use at 1200°C is often limited to just a few hours to prevent deformation and failure.
The Impact of Pressure
These transformation temperatures are defined at standard atmospheric pressure. The presence of high pressure can significantly alter the temperatures at which these phase transitions occur.
Making the Right Choice for Your Application
Your operational temperature dictates which properties of quartz are most important to consider.
- If your primary focus is on processes below 500°C: You are working exclusively with α-quartz and do not need to account for phase transitions.
- If your primary focus is on applications between 600°C and 850°C: You are operating in the stable β-quartz range, but must have accounted for the one-time expansion during the initial heat-up past 573°C.
- If your primary focus is on extreme temperatures above 870°C: You must consider not only the slow formation of tridymite but, more importantly, the practical softening point of the material to avoid catastrophic equipment failure.
Understanding these distinct thermal behaviors is the key to using quartz effectively and safely in any high-temperature environment.
Summary Table:
| Quartz Phase | Stability Temperature Range | Key Characteristics |
|---|---|---|
| α-Quartz (Alpha) | Up to 573°C (1063°F) | Stable, standard form at room temperature. |
| β-Quartz (Beta) | 573°C to ~870°C | Reversible phase change; displacive transformation. |
| Tridymite | Above ~870°C | Slow, reconstructive transformation; often irreversible. |
| Softening Point | ~1270°C (2318°F) | Material begins to deform; critical limit for structural integrity. |
Need precise, high-temperature lab equipment? The right furnace or quartz component is critical for your research's success and safety. KINTEK specializes in high-performance lab equipment and consumables, ensuring your materials can withstand the exact thermal phases described. Let our experts help you select the perfect solution for your application. Contact KINTEK today for a consultation!
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the technical significance of selecting hardened stainless steel grinding balls? Optimize Energy and Purity
- What is the purpose of using SiC grinding consumables for LZP electrolytes? Optimize Solid-State Battery Interfaces
- Why Use High-Purity Alumina Protection Tubes for S-Type Thermocouples? Prevent Contamination and Ensure Precision
- Why are oil-free vacuum pumps suitable for applications requiring a high level of cleanliness? Essential for Purity and Process Integrity
- How are sputtering targets used? Achieve Superior Thin-Film Coatings for Your Products
- What role does a dual-blade stirring mechanism play during the processing of Chromel-TaC melt? Ensure Homogeneity.
- What is the role of corundum tubes in oxygen permeation testing? Ensure Integrity for Bi-doped Membranes
- Why are high-density grinding media used for magnesium-based amorphous powders? Master Kinetic Energy Transfer



















