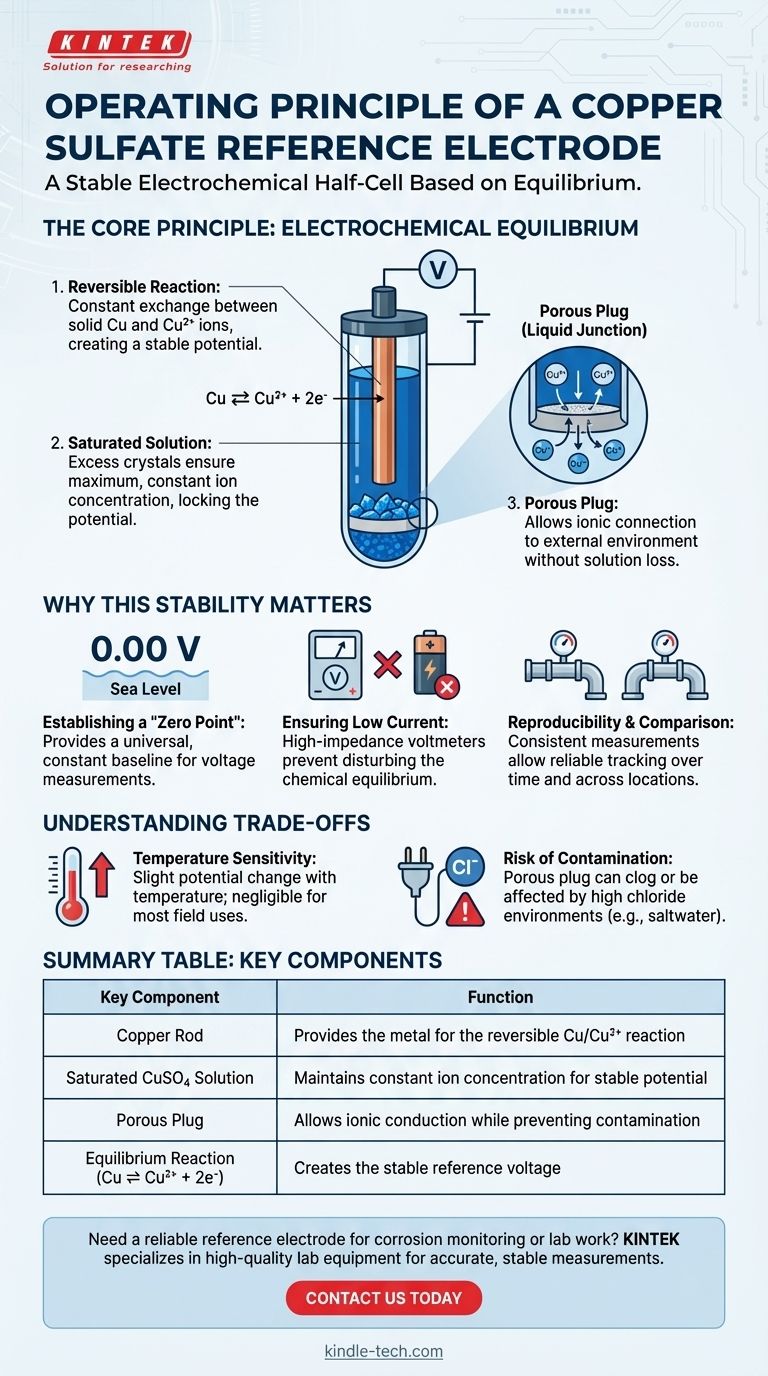
A copper sulfate reference electrode operates on a simple, reversible electrochemical reaction. It generates a highly stable and predictable voltage by immersing a pure copper rod into a saturated solution of copper(II) sulfate. This equilibrium between the solid copper metal and the copper ions in the solution creates a constant potential, which serves as a reliable benchmark for measuring the potential of other materials.
The fundamental purpose of a reference electrode is to provide an unwavering voltage baseline. The copper/copper sulfate system achieves this through a well-defined chemical equilibrium that remains stable as long as the solution is saturated, allowing for consistent and comparable potential measurements in other systems.

The Core Principle: A State of Equilibrium
The Electrochemical Half-Cell
To measure the electrical potential of any material, you need a complete circuit. The material you are testing (like a steel pipeline in the soil) acts as one half-cell.
The copper sulfate reference electrode provides the other, stable half-cell. By connecting a voltmeter between the two, you can measure the potential difference between them.
The Reversible Reaction
The stability of the electrode comes from a constant, reversible reaction at the surface of the copper rod:
Cu ⇌ Cu²⁺ + 2e⁻
This means that at any given moment, a tiny amount of solid copper (Cu) is dissolving into the solution as copper ions (Cu²⁺), releasing two electrons (2e⁻). Simultaneously, an equal number of copper ions are taking two electrons and plating back onto the rod as solid copper. This perfect balance is called equilibrium.
The Role of Saturation
The potential of this reaction depends on the concentration of copper ions in the solution. To keep the potential constant, the concentration of these ions must be kept constant.
This is achieved by using a saturated solution, meaning it contains the maximum possible amount of dissolved copper sulfate, often with undissolved crystals present. As long as solid crystals remain, the solution is guaranteed to be saturated, locking the ion concentration at a fixed value and thus locking in the electrode's potential.
The Porous Plug (Liquid Junction)
The electrode is sealed at the bottom with a porous plug made of wood or ceramic. This liquid junction is critical.
It allows ions to flow between the electrode's internal solution and the external environment (like soil or water), which is necessary to complete the electrical circuit for a measurement. However, it prevents the copper sulfate solution from quickly leaking out or becoming contaminated.
Why This Stability Matters in Practice
Establishing a "Zero Point"
A reference electrode acts like "sea level" for electrical potential. It provides a universally agreed-upon zero point.
When a voltmeter reads -0.85 V on a pipeline relative to a copper sulfate electrode, you are really measuring the difference between the two. Because the electrode's potential is known and constant, you can be confident that the reading reflects the true state of the pipeline.
Ensuring Low Current Flow
Measurements are taken with a high-impedance voltmeter. This is crucial because it draws almost no electrical current from the system.
If significant current were to flow through the reference electrode, it would disturb the chemical equilibrium, causing its potential to change and making the measurement invalid. The system is designed to be observed, not disturbed.
Reproducibility and Comparison
Because the potential of a copper sulfate electrode is so well-defined, measurements are highly reproducible.
A corrosion reading taken on a pipeline in Texas can be reliably compared to one taken in Ohio years later, providing a consistent standard for assessing structural integrity over time.
Understanding the Trade-offs
Temperature Sensitivity
While very stable, the electrode's potential has a slight dependence on temperature. For most field applications this is negligible, but for high-precision laboratory work, temperature must be recorded and accounted for.
Risk of Contamination
The porous plug can become clogged over time. More importantly, if used in environments with high chloride concentrations (like saltwater), chloride ions can seep into the electrode and alter its reference potential, leading to inaccurate readings.
Designed for the Field
The physical design—often a transparent, robust body with a screw-cap for refilling—is a direct result of its primary use. It is built to be durable, portable, and easily maintained for on-site industrial testing, particularly for cathodic protection systems on buried structures.
Making the Right Choice for Your Goal
The best reference electrode is the one most suited to its environment.
- If your primary focus is field-based corrosion monitoring (e.g., pipelines or tanks in soil): The copper/copper sulfate electrode is the industry standard due to its robustness, stability, and low cost.
- If you are working in an environment with high chloride content (e.g., seawater or reinforced concrete): A silver/silver chloride (Ag/AgCl) electrode is a better choice, as its chemistry is more stable in the presence of chlorides.
- If you require high precision in a controlled laboratory setting: A saturated calomel electrode (SCE) has historically been a standard, though it contains mercury and is less common today for field use.
Understanding the principle of a stable equilibrium is the key to trusting your electrochemical measurements and making informed decisions based on them.
Summary Table:
| Key Component | Function |
|---|---|
| Copper Rod | Provides the metal for the reversible Cu/Cu²⁺ reaction |
| Saturated CuSO₄ Solution | Maintains constant ion concentration for stable potential |
| Porous Plug | Allows ionic conduction while preventing contamination |
| Equilibrium Reaction (Cu ⇌ Cu²⁺ + 2e⁻) | Creates the stable reference voltage |
Need a reliable reference electrode for your corrosion monitoring or lab work? KINTEK specializes in high-quality lab equipment and consumables, including robust reference electrodes designed for accurate, stable measurements in the field or laboratory. Our experts can help you select the right electrode for your specific environment and application. Contact us today to discuss your needs and ensure the integrity of your electrochemical data!
Visual Guide
Related Products
- Copper Sulfate Reference Electrode for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Metal Disc Electrode Electrochemical Electrode
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- What are the advantages and disadvantages of the wood plug type copper sulfate reference electrode? Speed vs. Durability Explained
- Is there a difference in performance between wood plug and ceramic core copper sulfate electrodes? Speed vs. Durability Explained
- What is the expected lifespan of a copper sulfate reference electrode? Maximize Longevity with Proper Maintenance
- What precautions should be taken when handling and using a copper sulfate reference electrode? Ensure Accurate Electrochemical Measurements
- What are the pre-treatment steps before using a portable copper sulfate reference electrode? Ensure Accurate Corrosion Potential Measurements



















