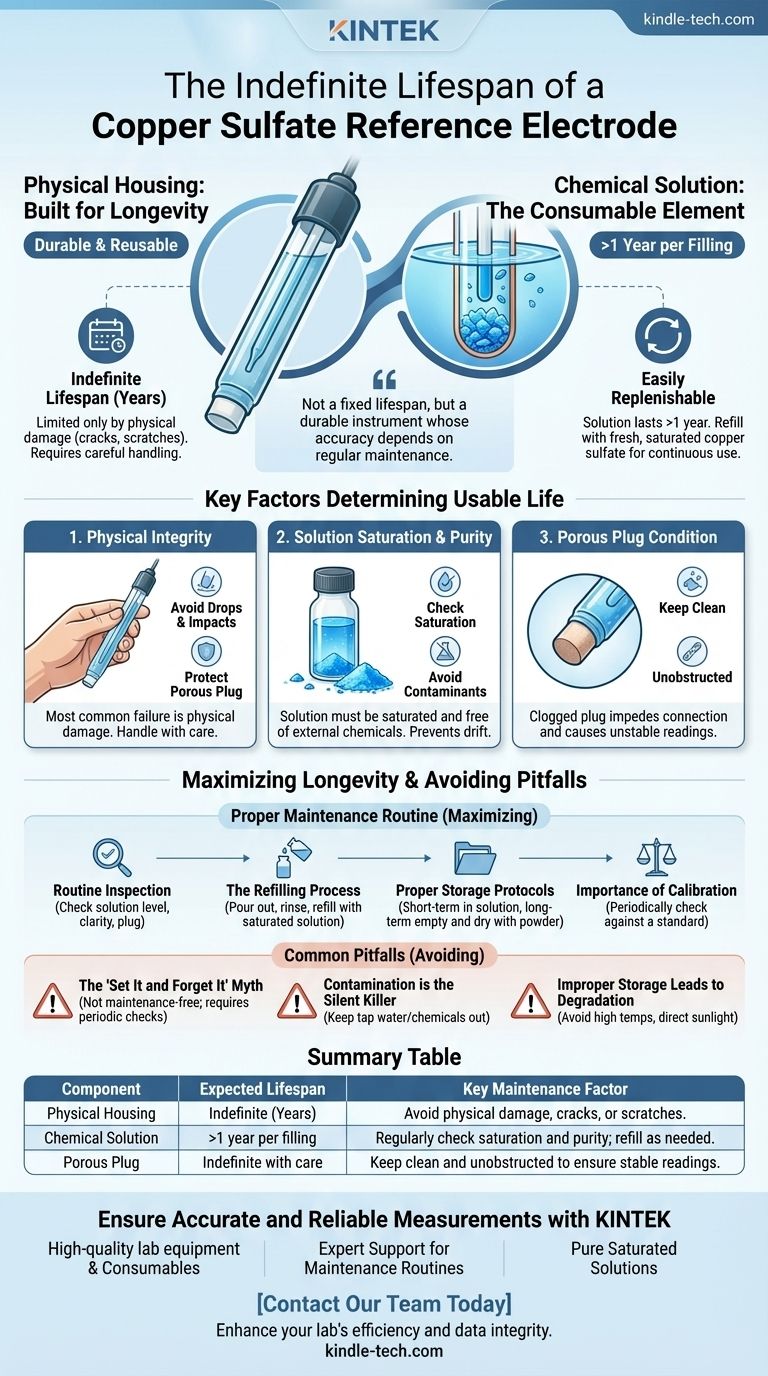
The physical body of a copper sulfate reference electrode is designed for an indefinite lifespan, limited only by physical damage. Its operational life, however, is determined by the internal copper sulfate solution, which typically lasts for more than a year per filling and can be easily replenished for continuous long-term use.
The concept of a fixed "lifespan" for a copper sulfate electrode is misleading. It's better understood as a durable, reusable instrument whose accuracy and function depend on the regular maintenance of its consumable chemical solution, not on a predetermined expiration date.

The Two Components of Electrode Lifespan
An electrode's longevity is best understood by separating its two primary components: the physical housing and the chemical solution.
The Physical Housing: Built for Longevity
The main body of the electrode, often made of transparent organic glass, is designed to be robust and reusable.
As long as this chamber remains free of cracks, scratches, or other physical damage, it can be used for many years. The critical failure point is physical damage, not material degradation over time.
The Chemical Solution: The Consumable Element
The saturated copper sulfate solution inside the electrode is the active, consumable component.
A single, fresh filling of this solution can provide a stable reference potential for over a year. The transparent body allows for easy visual inspection to confirm the solution remains saturated and free of contaminants.
Key Factors That Determine Usable Life
The practical service life of the electrode is not a matter of time, but of condition. Three factors are paramount.
Physical Integrity
The single most common reason for permanent electrode failure is physical damage.
Avoiding drops, impacts, and squeezing is critical. Special care must be taken to protect the microporous membrane or ceramic plug at the tip, as scratches or clogs will disrupt its function.
Solution Saturation and Purity
The electrode's stability is directly tied to the state of its internal solution.
The solution must remain saturated with copper sulfate crystals and be free from external contaminants. If the solution becomes diluted or contaminated, the reference potential will drift, leading to inaccurate measurements.
Porous Plug Condition
The porous plug provides the liquid junction with the test environment.
If this plug becomes clogged with dirt, debris, or precipitates, it will impede the electrochemical connection and render the readings unstable or incorrect.
Understanding the Common Pitfalls
Proper care is straightforward, but neglecting it will compromise the electrode's performance and shorten its usable life.
The "Set It and Forget It" Myth
This is not a maintenance-free device. It requires periodic inspection of the solution level, clarity, and saturation. Regular checks prevent minor issues from becoming sources of significant measurement error.
Contamination Is the Silent Killer
Allowing external chemicals or even tap water to mix with the internal solution can irreversibly alter its potential. This often happens through improper cleaning or a compromised seal.
Improper Storage Leads to Degradation
Storing the electrode incorrectly is a primary cause of premature failure. High temperatures, direct sunlight, and significant temperature fluctuations can degrade the solution and the electrode's physical components.
Maximizing Longevity Through Proper Maintenance
A simple maintenance routine ensures the electrode remains a reliable and long-lasting tool.
Routine Inspection and Cleaning
Periodically wipe the outer casing with a soft, damp cloth. Most importantly, inspect the porous plug to ensure it is clean and unobstructed. Check the solution level and look for signs of contamination.
The Refilling Process
The screw-threaded cap is specifically designed for easy maintenance. When the solution is depleted or contaminated, it can be poured out, the chamber rinsed with distilled water, and refilled with fresh, saturated copper sulfate solution.
Proper Storage Protocols
For short-term storage, keep the electrode tip immersed in a separate container of saturated copper sulfate solution. For long-term storage, empty the solution, rinse the chamber with distilled water, allow it to dry, and refill it with dry copper sulfate powder.
The Importance of Calibration
Periodically measure the electrode's output in a solution of known potential. A significant deviation signals that the internal solution needs to be replaced or the electrode requires cleaning, long before it affects your critical field measurements.
A Practical Approach to Electrode Lifespan
Your maintenance strategy should align with your operational priorities.
- If your primary focus is maximizing equipment life: Prioritize careful handling above all else to prevent physical damage and implement a consistent schedule for inspection and cleaning.
- If your primary focus is measurement accuracy: Regularly calibrate the electrode and never hesitate to refresh the copper sulfate solution if you suspect contamination or depletion.
- If your primary focus is cost-efficiency: Leverage the electrode's refillable design by properly performing long-term storage procedures during periods of inactivity to preserve the instrument.
Ultimately, a copper sulfate reference electrode, when properly maintained, is not a disposable item but a long-term, reliable instrument in your toolkit.
Summary Table:
| Component | Expected Lifespan | Key Maintenance Factor |
|---|---|---|
| Physical Housing | Indefinite (Years) | Avoid physical damage, cracks, or scratches. |
| Chemical Solution | >1 year per filling | Regularly check saturation and purity; refill as needed. |
| Porous Plug | Indefinite with care | Keep clean and unobstructed to ensure stable readings. |
Ensure Accurate and Reliable Measurements with KINTEK
A well-maintained copper sulfate reference electrode is crucial for precise electrochemical measurements. At KINTEK, we specialize in providing high-quality lab equipment and consumables, including durable reference electrodes and the necessary copper sulfate solutions for maintenance.
Our experts can help you:
- Select the right electrode for your specific application.
- Establish a proactive maintenance routine to maximize instrument lifespan.
- Source pure, saturated copper sulfate solutions for reliable refills.
Don't let electrode maintenance compromise your data integrity. Contact our team today to discuss your laboratory needs and discover how KINTEK's products and support can enhance your lab's efficiency and accuracy.
Visual Guide
Related Products
- Copper Sulfate Reference Electrode for Laboratory Use
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Metal Disc Electrode Electrochemical Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
People Also Ask
- What are the advantages and disadvantages of the wood plug type copper sulfate reference electrode? Speed vs. Durability Explained
- How should a copper sulfate reference electrode be stored? A Guide to Short-Term & Long-Term Storage
- How should a copper sulfate reference electrode be maintained? Ensure Accurate Electrochemical Measurements
- What is the operating principle of a copper sulfate reference electrode? Reliable Potential Measurement Explained
- What are the advantages and disadvantages of the ceramic core type copper sulfate reference electrode?



















