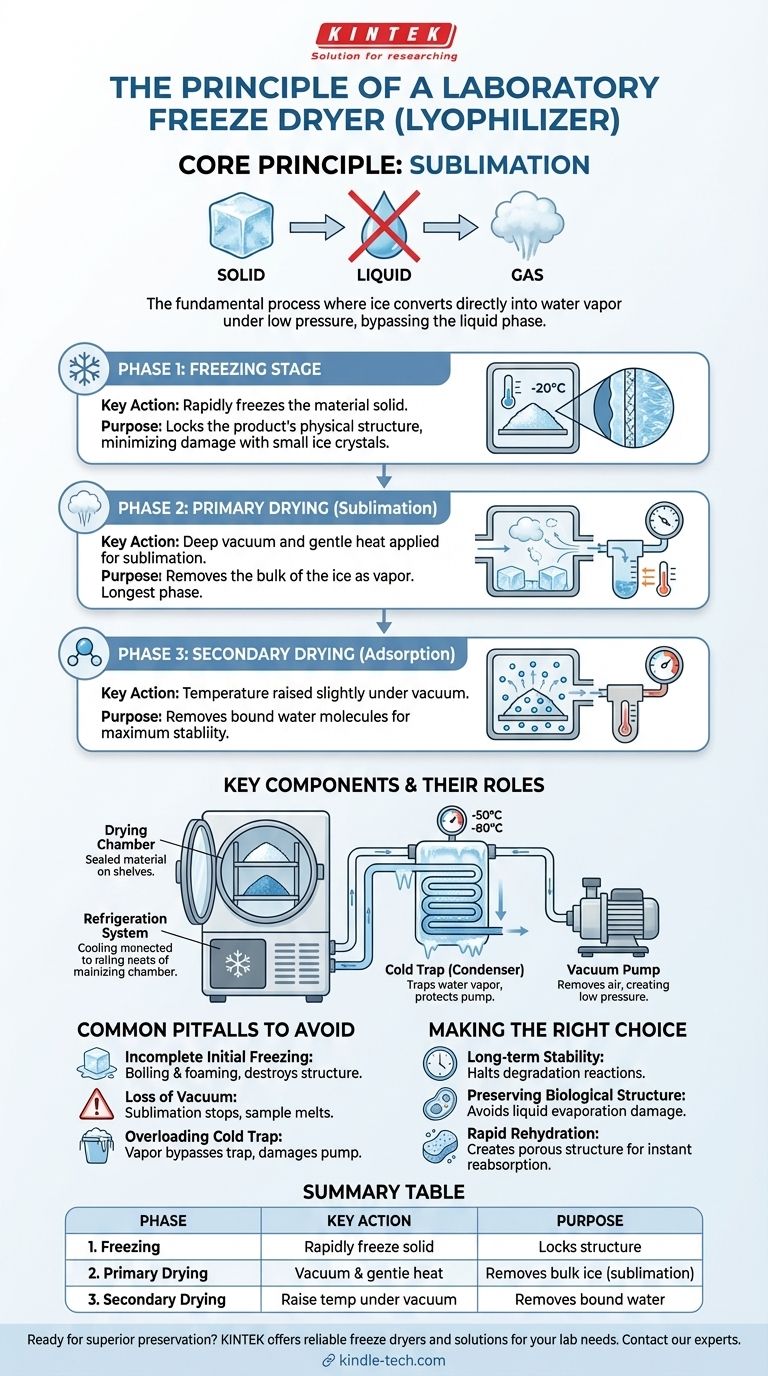
The fundamental principle of a laboratory freeze dryer is sublimation. This is a physical process where a solid, in this case, ice, is converted directly into a gas (water vapor) without passing through the liquid water phase. The entire system is engineered to create the specific low-pressure and low-temperature conditions required for this transition to occur gently and efficiently.
A freeze dryer, or lyophilizer, doesn't simply remove water; it preserves a material's delicate structure and chemical integrity. It achieves this through a controlled, three-phase process: freezing the material solid, then using a deep vacuum to turn the ice directly into vapor, and finally removing any remaining bound moisture.

The Three-Phase Process of Lyophilization
Freeze drying is not a single event but a carefully orchestrated sequence. Each phase has a distinct purpose in achieving a stable, dry product.
Phase 1: The Freezing Stage
The initial step is to completely freeze the material. This is a critical stage because it locks the product's physical structure into a solid state.
The goal is typically to freeze the material rapidly to form small ice crystals, which minimizes potential damage to the cellular or molecular structure of the sample.
Phase 2: Primary Drying (Sublimation)
This is the core of the freeze-drying process and the longest phase. After freezing, a deep vacuum is applied to the chamber.
This drastic reduction in pressure lowers the boiling point of water. A small amount of heat is then carefully introduced, providing just enough energy for the frozen water molecules to break free and sublimate directly into vapor.
This water vapor is then drawn away from the sample and collected on an extremely cold surface called a cold trap or condenser.
Phase 3: Secondary Drying (Adsorption)
After primary drying has removed the bulk of the ice, a small amount of unfrozen water molecules may still be chemically bound to the material.
In this final phase, the temperature is raised slightly higher while the vacuum is maintained. This provides the energy needed to break the bonds holding these last water molecules, which are then also removed and trapped by the condenser, ensuring the final product is maximally dry and stable.
Key Components and Their Roles
Understanding the core machinery demystifies the process and highlights how each part enables sublimation.
The Drying Chamber
This is the sealed container that holds the material being dried. It is where the vacuum is applied and temperature is controlled.
The Cold Trap (Condenser)
The cold trap is the unsung hero of the system. It is a surface refrigerated to a temperature significantly colder than the material (e.g., -50°C to -80°C).
Its sole purpose is to attract and trap the water vapor released during sublimation, freezing it back into solid ice. This protects the vacuum pump from corrosive water vapor and helps maintain the low pressure in the chamber.
The Vacuum Pump
The vacuum pump is responsible for removing air from the drying chamber and cold trap. This creates the low-pressure environment that is essential for sublimation to occur at low temperatures.
The Refrigeration System
This system has two primary jobs. First, it freezes the material in the initial phase. Second, and more importantly, it continuously cools the cold trap to ensure it remains effective at capturing water vapor throughout the drying process.
Common Pitfalls to Avoid
The precision of the process means that small errors can have significant consequences for the final product.
Incomplete Initial Freezing
If the material is not frozen completely solid before the vacuum is applied, it will simply boil and foam under vacuum, destroying the sample's structure.
Loss of Vacuum
A leak in the system or a failing pump will cause the pressure to rise. If this happens, the sublimation process will stop, and the frozen sample can melt, leading to product failure.
Overloading the Cold Trap
Attempting to dry a volume of liquid that exceeds the capacity of the cold trap will overwhelm it. Water vapor will bypass the trap and enter the vacuum pump, causing damage and compromising the vacuum level.
Making the Right Choice for Your Goal
Understanding the principle of sublimation allows you to leverage freeze drying for specific scientific and practical outcomes.
- If your primary focus is long-term stability: Freeze drying is the ideal method, as removing water halts nearly all enzymatic and chemical reactions that cause degradation.
- If your primary focus is preserving biological structure: Sublimation avoids the surface tension forces of liquid water evaporation, which would otherwise shrink and damage delicate structures like cells or proteins.
- If your primary focus is rapid rehydration: The process creates a porous, sponge-like structure (a "lyophile") that allows water to be reabsorbed almost instantly, restoring the material to its original state.
Ultimately, freeze drying is a sophisticated technique for gently removing water, making it the gold standard for preserving sensitive biologicals, pharmaceuticals, and foods.
Summary Table:
| Phase | Key Action | Purpose |
|---|---|---|
| 1. Freezing | Rapidly freeze the material solid | Locks the sample's structure in place |
| 2. Primary Drying | Apply vacuum & gentle heat for sublimation | Removes bulk ice directly as vapor |
| 3. Secondary Drying | Raise temperature under vacuum | Removes bound water molecules for stability |
Ready to achieve superior sample preservation with a freeze dryer? KINTEK specializes in providing reliable lab equipment and consumables for all your laboratory needs. Our freeze dryers are designed to deliver precise control for optimal results with your sensitive biologicals, pharmaceuticals, and research materials. Contact our experts today to find the perfect lyophilization solution for your lab!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- Why is a laboratory freeze-drying system essential for fermentation biomass? Preserve Sample Integrity for Analysis
- What role does a laboratory freeze dryer play in the synthesis of graphene-based electrocatalysts? Preserve 3D Structures
- Why is a laboratory vacuum freeze dryer essential for plant extracts? Preserve Bioactivity & Structure
- What is the function of a freeze dryer in the ice-templating process? Preserving Aligned Pore Scaffolds for LAGP
- Why is a freeze dryer preferred for drying nickel nanoparticle precursors? Prevent Hard Agglomeration Now



















