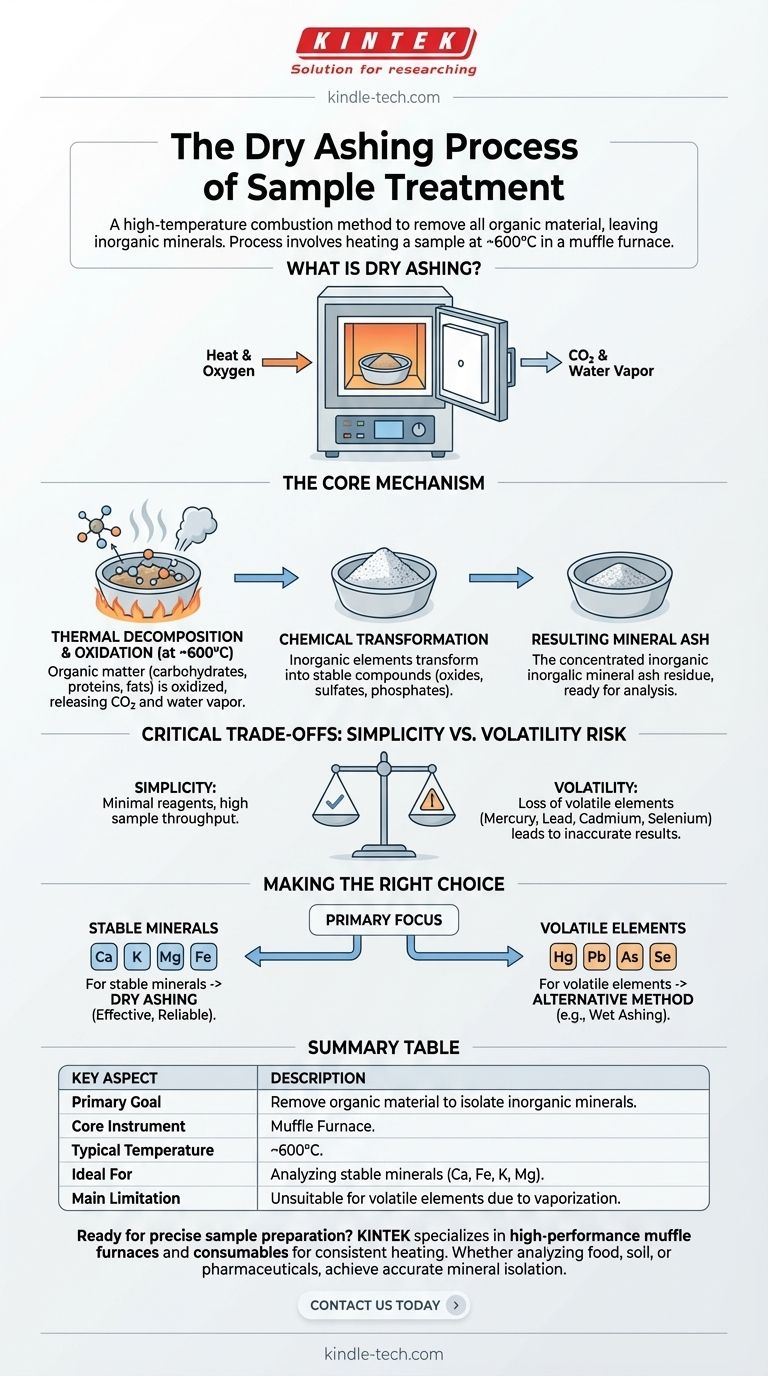
In essence, dry ashing is a high-temperature combustion method used to remove all organic material from a sample, leaving behind only the inorganic, mineral components for analysis. The process involves heating a sample in a specialized high-temperature oven, called a muffle furnace, to temperatures around 600°C until only a mineral ash residue remains.
Dry ashing is a powerful technique for isolating the total mineral content of a sample, but its high-temperature nature creates a critical trade-off: simplicity and effectiveness come at the risk of losing volatile minerals, which can lead to inaccurate results.

The Core Mechanism of Dry Ashing
The Role of the Muffle Furnace
A muffle furnace is the primary instrument used for dry ashing. It provides a precisely controlled, high-temperature environment necessary for complete combustion.
The furnace heats the sample, typically held in a ceramic or porcelain crucible, driving off water and systematically burning away the organic matrix.
Thermal Decomposition and Oxidation
As the temperature rises, two key processes occur. First, water and other volatile compounds evaporate.
Then, at approximately 600°C, the organic matter (like carbohydrates, proteins, and fats) is completely oxidized—it reacts with oxygen in the air and is converted into carbon dioxide and water vapor, which are released.
Chemical Transformation of Minerals
The inorganic elements that remain are not in their original form. The intense heat transforms them into more stable compounds like oxides, sulfates, and phosphates.
This resulting ash is a concentrated sample of the original material's mineral content, ready for further quantitative analysis.
Understanding the Critical Trade-offs
The Primary Limitation: Volatility
The main disadvantage of dry ashing is the potential loss of volatile elements. Minerals like mercury, lead, cadmium, and selenium can vaporize at the high temperatures used in the process.
This loss leads to an underestimation of these elements in the final analysis, producing an inaccurate outcome. The method is therefore unsuitable for samples where these specific elements are of interest.
Simplicity vs. Accuracy
Dry ashing is often preferred for its simplicity, as it requires minimal reagents and can process many samples at once. However, this simplicity must be weighed against the potential for inaccuracy.
If a sample is known to contain volatile minerals, a lower-temperature method or a different technique like wet ashing may be required to ensure accurate results.
Making the Right Choice for Your Analysis
Choosing the correct sample preparation method depends entirely on your analytical goals.
- If your primary focus is on stable, non-volatile minerals (e.g., calcium, potassium, magnesium, iron): Dry ashing is a highly effective, simple, and reliable method.
- If your primary focus is on volatile or trace elements (e.g., mercury, lead, arsenic, selenium): Dry ashing is unsuitable and will produce inaccurate data; you must use an alternative method like wet ashing.
Ultimately, understanding the fundamental principles and limitations of dry ashing is the key to generating reliable and meaningful analytical data.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Goal | Remove organic material to isolate inorganic mineral content for analysis. |
| Core Instrument | Muffle Furnace. |
| Typical Temperature | ~600°C. |
| Ideal For | Analyzing stable minerals like calcium, iron, potassium, and magnesium. |
| Main Limitation | Unsuitable for volatile elements (e.g., mercury, lead, selenium) due to vaporization. |
Ready to achieve precise and reliable sample preparation?
The right equipment is critical for accurate dry ashing. KINTEK specializes in high-performance muffle furnaces and lab consumables designed for consistent, high-temperature heating. Whether you're analyzing food, soil, or pharmaceuticals, our solutions help you isolate mineral content with confidence.
Contact us today to discuss your laboratory's specific needs and let our experts help you select the perfect equipment for your application.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the function of a high-temperature muffle furnace in ZnO nanocatalyst synthesis? Ensure Pure Crystallinity
- What role does a muffle furnace play in Sm-doped SrTiO3 ceramics? Ensure Phase Purity and Precision Synthesis
- What is the primary function of an electric resistance furnace in TCT? Master 12Kh18N10T Steel Microstructure Recovery
- Why is a high-precision convection drying oven necessary for TiO2/ZnO catalysts? Stabilize Your Material Pore Structure
- What is the calcination process? A Guide to Thermal Purification and Material Transformation
- What is the purpose of using vacuum ovens for degassing Alumina/MWNT composites? Ensure Flawless Structural Integrity
- What does 'sintered' mean and why is it important to understand? Unlock Advanced Materials & Manufacturing
- How does a high-temperature air oxidation furnace achieve rapid thickening of the oxide layer on Zircaloy-4?



















