In a standard laboratory-grade platinum sheet electrode, the platinum sheet itself has a purity of 99.99%. This high grade, often referred to as "four nines" purity, is not a luxury but a fundamental requirement. It is the key factor that ensures the electrode remains inert and performs predictably, thereby guaranteeing the accuracy and reliability of experimental data.
The 99.99% purity of a platinum electrode is essential because electrochemistry is a science of surfaces. Any impurity, even at a trace level, can introduce unwanted side reactions or alter the electrode's catalytic behavior, compromising the integrity of your results.
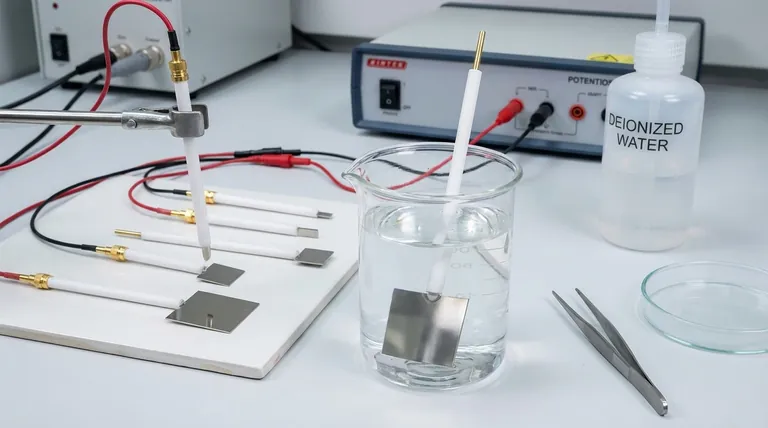
Why Purity is a Non-Negotiable Requirement
The function of a platinum electrode is to facilitate electron transfer without interfering with the chemical system under study. Its high purity is central to achieving this goal.
To Ensure Chemical Inertness
Platinum is valued for its exceptional stability. It resists corrosion and reaction in strong acids, strong alkalis, and other aggressive electrolytes.
This inertness is directly tied to its purity. Impurities, such as other metals, could leach into your electrolyte or react at certain potentials, creating electrochemical signals that contaminate your data and lead to incorrect conclusions.
To Guarantee Consistent Catalytic Performance
Platinum is an excellent catalyst for many important electrochemical reactions, including the hydrogen evolution reaction (HER) and the oxygen reduction reaction (ORR).
Impurities on the electrode surface can "poison" the catalyst, blocking active sites or changing the reaction mechanism entirely. Using 99.99% pure platinum ensures that the catalytic activity you measure is characteristic of platinum itself, making your results comparable and reproducible.
To Maintain a Wide, Stable Potential Window
A key advantage of platinum is its ability to operate across a broad range of electrical potentials without degrading.
This wide operational window allows researchers to study a variety of redox reactions. The high purity ensures that the electrode's physical and chemical structure remains stable even at extreme positive or negative potentials.
Common Pitfalls and Handling Protocols
While robust, the high-purity nature of a platinum electrode makes it susceptible to contamination if handled improperly. Its effectiveness is as much a result of proper care as it is of its material composition.
The Risk of Surface Contamination
The primary risk is not from the bulk material but from substances adsorbing onto the platinum surface. Oils from your hands, residues from previous experiments, or airborne particles can all alter the electrode's behavior.
This is why strict cleaning protocols are not just suggestions; they are mandatory for good scientific practice.
The Importance of Correct Immersion
When setting up your experiment, it is imperative that only the platinum sheet comes into contact with the electrolyte.
Submerging the connecting wire or the encapsulating material (often glass or PTFE) can introduce contaminants or create an alternative electrical pathway, invalidating your measurements.
Post-Experiment Cleaning and Storage
The life and reliability of your electrode depend on what you do immediately after an experiment concludes.
Rinse the electrode thoroughly with deionized water to remove all traces of electrolyte. After it is dry, store it in a clean, protective case with the platinum sheet facing upwards to prevent mechanical damage or scratches to the active surface.
Making the Right Choice for Your Goal
Understanding the properties of your platinum electrode allows you to use it effectively to achieve your specific experimental aims.
- If your primary focus is quantitative analysis: The 99.99% purity is your guarantee against interference, ensuring that the currents you measure are solely from your analyte of interest.
- If your primary focus is catalysis research: This purity level is non-negotiable, as it provides a clean, predictable surface to study intrinsic reaction kinetics without ambiguity.
- If your primary focus is instrument longevity and reproducibility: Adhering to strict cleaning and storage protocols is just as critical as the initial purity for obtaining consistent results over time.
Ultimately, recognizing that a platinum electrode is a precision instrument, not just a piece of metal, is the foundation for generating trustworthy and repeatable electrochemical data.
Summary Table:
| Key Aspect | Specification/Requirement |
|---|---|
| Platinum Purity | 99.99% (Four Nines Grade) |
| Primary Function | Facilitate electron transfer without interference |
| Key Benefit | Ensures chemical inertness and predictable performance |
| Critical Handling | Avoid surface contamination; proper immersion and cleaning |
Ensure the integrity of your electrochemical research with high-purity platinum electrodes from KINTEK.
KINTEK specializes in premium lab equipment and consumables, providing researchers with the reliable tools needed for accurate and reproducible data. Our high-purity platinum electrodes are designed to meet the stringent demands of quantitative analysis and catalysis studies.
Contact our experts today to discuss how our electrodes can enhance your laboratory's performance and support your scientific goals.
Visual Guide

Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Electrochemical Sheet Electrode Gold Electrode
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- What is the proper post-treatment procedure for a platinum sheet electrode? Ensure Long-Term Accuracy & Protect Your Investment
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs
- What is the expected lifespan of a platinum sheet electrode? Maximize Your Electrode's Service Life
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results



















