At its core, sintering is a high-temperature thermal process that transforms a fragile, compacted ceramic powder into a solid, dense, and mechanically robust component. It is the critical manufacturing step where individual ceramic particles are heated below their melting point, causing them to bond together and eliminate the empty spaces between them, resulting in a strong, polycrystalline material.
The fundamental challenge in creating advanced ceramics is converting a loose powder into a high-performance solid. Sintering solves this by using controlled thermal energy to drive atomic diffusion, which fuses particles together and removes internal porosity, thereby dictating the final properties of the ceramic part.

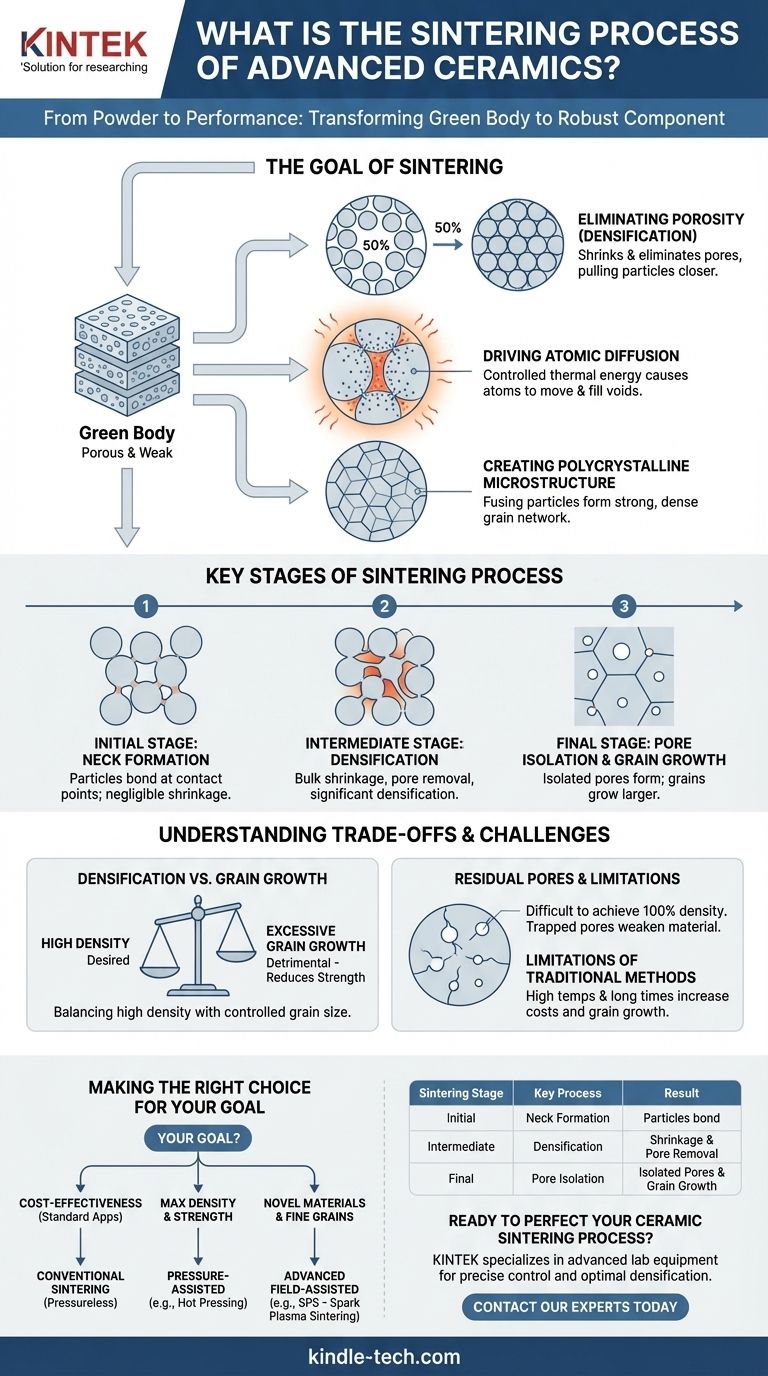
The Goal of Sintering: From Powder to Performance
The process begins with a "green body," which is ceramic powder that has been pressed or formed into a desired shape. This green body is porous and mechanically weak. Sintering is the essential step that converts it into a strong, functional ceramic.
Eliminating Porosity (Densification)
The primary objective of sintering is densification. The green body can contain up to 50% empty space, or porosity, by volume. These pores are defects that severely compromise the material's strength and performance. Sintering aims to shrink and eliminate these pores, pulling the ceramic particles closer together.
Driving Atomic Diffusion
Sintering works by promoting atomic diffusion. The high temperatures in a sintering furnace give atoms at the surfaces of the ceramic particles enough energy to move. Atoms migrate from the bulk of the particles to the points of contact between them, gradually filling the voids. This is the mechanism that causes the particles to fuse and the overall part to densify.
Creating a Polycrystalline Microstructure
As the particles fuse, they form a dense, interconnected network of crystalline grains. This resulting polycrystalline microstructure is what gives the final ceramic part its characteristic hardness, strength, and thermal stability. The size and uniformity of these grains are critical to the material's performance.
Key Stages of the Sintering Process
Sintering is not instantaneous; it progresses through distinct stages, each contributing to the final microstructure.
Initial Stage: Neck Formation
When the temperature rises, the first points of contact between adjacent particles begin to fuse. This creates small bridges or "necks" between them. During this stage, the overall part does not shrink significantly, but the particles become bonded together.
Intermediate Stage: Densification
As the necks grow larger, they pull the particle centers closer together. This causes the bulk component to shrink and become more dense. The pores, which were previously interconnected, form a network of cylindrical channels running through the structure. The most significant densification occurs during this stage.
Final Stage: Pore Isolation and Grain Growth
In the final stage, the pore channels break apart and become isolated, spherical voids. These isolated pores are much more difficult to remove and can become trapped within the growing grains. It is also at this stage that grain growth accelerates, a phenomenon that can be detrimental to mechanical properties if not controlled.
Understanding the Trade-offs and Challenges
Achieving a perfect sintered body requires balancing competing phenomena. The success of the process depends on carefully controlling key parameters.
Densification vs. Grain Growth
The most critical trade-off in sintering is between achieving high density and preventing excessive grain growth. The same high temperatures and long times that promote densification also cause the crystalline grains to grow larger. Overly large grains can reduce the material's strength and fracture toughness.
The Problem of Residual Pores
It is extremely difficult to achieve 100% theoretical density. Residual pores, especially those trapped inside grains during the final stage, act as stress concentrators. Under mechanical load, these pores can become the initiation points for cracks, leading to catastrophic failure.
Limitations of Traditional Methods
Traditional, or pressureless, sintering involves simply heating the green body in a furnace. For many advanced ceramics, this method requires very high temperatures and long hold times, which increases energy costs and exacerbates the problem of unwanted grain growth.
Making the Right Choice for Your Goal
The choice of sintering method depends entirely on the material being processed and the desired final properties of the component.
- If your primary focus is cost-effectiveness for standard applications: Conventional, pressureless sintering is often sufficient and is the most economical choice.
- If your primary focus is achieving maximum density and mechanical strength: Pressure-assisted methods, like hot pressing, are superior as the applied pressure aids in pore closure at lower temperatures.
- If your primary focus is processing novel materials or achieving ultra-fine grain structures: Advanced field-assisted techniques, such as Spark Plasma Sintering (SPS), are necessary to heat rapidly and minimize grain growth.
Mastering the sintering process is the key to unlocking the full performance potential of any advanced ceramic material.
Summary Table:
| Sintering Stage | Key Process | Result |
|---|---|---|
| Initial Stage | Neck Formation | Particles bond at contact points |
| Intermediate Stage | Densification | Significant shrinkage and pore removal |
| Final Stage | Pore Isolation & Grain Growth | Isolated pores form; grains grow |
Ready to perfect your ceramic sintering process? KINTEK specializes in providing the advanced lab equipment and consumables needed to achieve precise temperature control and optimal densification for your laboratory's materials. Whether you're working with conventional or advanced ceramics, our solutions help you maximize performance and efficiency. Contact our experts today to discuss your specific sintering challenges and goals!
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Is silicon carbide a good electrical insulator? Discover Its Role as a High-Performance Semiconductor
- What are the process advantages of selecting an alumina plate for CuO nanofilm synthesis? Achieve Superior Purity
- What is the function of a ceramic liner in a reaction chamber? Enhance Data Precision in Steam Oxidation Testing
- What are the different types of ceramic styles? A Guide to Earthenware, Stoneware, Porcelain & Bone China
- What is the overview of ceramics? Unlocking the Potential of Advanced Materials
- What are the benefits of sintering ceramics? Unlock Superior Strength and Performance
- Is ceramic chemically inert? Unlock the Power of Ultimate Chemical Resistance
- What is the thermal resistance of SiC? Understanding Its High Thermal Conductivity for Superior Performance



















