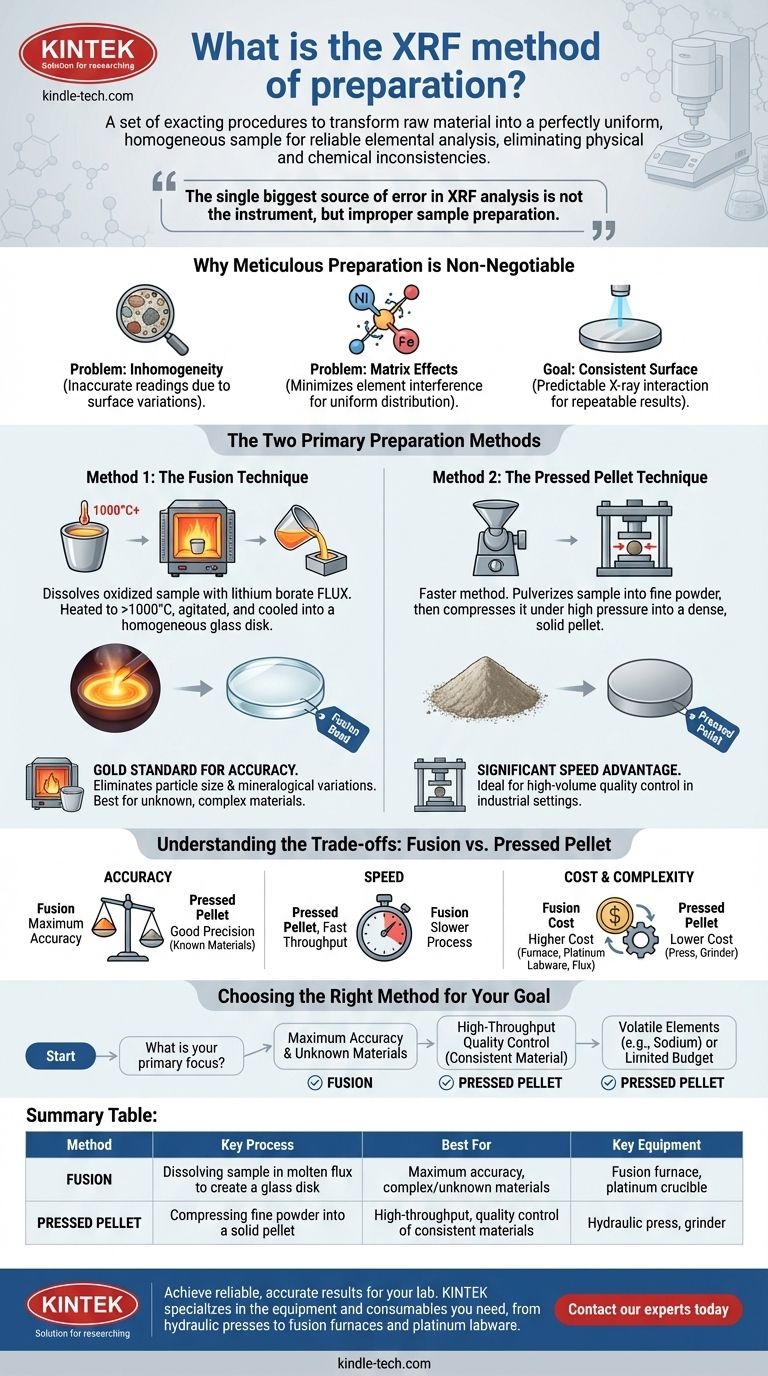
To be precise, the XRF method of preparation is a set of exacting procedures designed to transform a raw material into a perfectly uniform and homogeneous sample for elemental analysis. This typically involves either crushing the sample and pressing it into a solid pellet or dissolving it in a molten flux at high temperatures to create a flawless glass disk. The overarching goal is to eliminate physical and chemical inconsistencies that would otherwise produce inaccurate results.
The single biggest source of error in X-Ray Fluorescence (XRF) analysis is not the instrument, but improper sample preparation. The fundamental goal of any preparation method is to present a perfectly flat, dense, and chemically uniform surface to the analyzer.

Why Meticulous Preparation is Non-Negotiable
XRF analysis works by bombarding a sample with X-rays and measuring the secondary X-rays that are emitted back. The accuracy of this measurement is critically dependent on the physical and chemical state of the sample itself.
The Problem of Inhomogeneity
An XRF spectrometer analyzes a relatively small surface area of the sample. If the sample is not perfectly uniform—for instance, a rock with different mineral grains—the instrument will only read the composition of the specific spot it hits, which is not representative of the whole.
Eliminating "Matrix Effects"
The various elements within a sample can interfere with one another's signals, a phenomenon known as "matrix effects." A proper preparation technique, particularly fusion, minimizes these interferences by creating a new, uniform material where all elements are evenly distributed.
The Ultimate Goal: A Consistent Surface
Both major preparation methods aim to create a sample that is perfectly flat, dense, and has a consistent particle size and distribution. This ensures that the X-ray beam interacts with the sample predictably every single time, leading to repeatable and reliable results.
The Two Primary Preparation Methods
While there are variations, nearly all XRF preparation falls into two main categories: creating a fused bead or a pressed pellet.
Method 1: The Fusion Technique
Fusion is a highly effective method that involves completely dissolving the sample. The oxidized sample is mixed with a solvent, typically a lithium borate flux, inside a platinum crucible.
This mixture is heated in a furnace to over 1000°C until it becomes a molten liquid. The molten mixture is agitated and then poured into a mold to cool into a perfectly homogeneous glass disk, ready for analysis.
Method 2: The Pressed Pellet Technique
This method is generally faster and involves mechanical rather than chemical transformation. The sample is first pulverized into an extremely fine and consistent powder.
This powder is then placed into a die and compressed under immense pressure by a hydraulic press. This force compacts the powder into a dense, solid pellet with a smooth analytical surface. Sometimes a binding agent is mixed with the powder to improve the pellet's stability.
Understanding the Trade-offs: Fusion vs. Pressed Pellet
Choosing a method requires balancing the need for accuracy against practical constraints like time, cost, and sample type.
Accuracy and Reliability
Fusion is the gold standard for accuracy. By dissolving the sample, it virtually eliminates errors from particle size differences and mineralogical variations. It is the superior choice for creating calibration standards or analyzing unknown, complex materials.
Pressed pellets can be highly precise for known materials but are more susceptible to matrix effects. Results can be skewed if the particle size of the unknown sample differs from the calibration standards.
Speed and Throughput
Pressed pellets offer a significant speed advantage. The process of pulverizing and pressing is much faster than the heating, dissolving, and cooling cycle required for fusion. This makes it ideal for high-volume industrial environments like cement plants or mining operations.
Cost and Complexity
Fusion is more expensive. It requires specialized high-temperature furnaces, expensive platinum labware (crucibles and molds), and a continuous supply of high-purity flux.
The pressed pellet method requires a grinder and a hydraulic press, which represents a lower initial and ongoing investment compared to a full fusion setup.
Choosing the Right Method for Your Goal
Your analytical objective dictates the correct preparation strategy. An inconsistent or poorly chosen method will undermine the validity of your results, regardless of how advanced your spectrometer is.
- If your primary focus is maximum accuracy and analyzing diverse or unknown materials: Fusion is the only method that reliably eliminates the complex matrix effects required for trustworthy results.
- If your primary focus is high-throughput quality control of a consistent material: The pressed pellet method provides the speed necessary for process control and can deliver excellent precision when properly calibrated.
- If you are analyzing for volatile elements (like sodium) or working with a limited budget: The lower temperatures and lower cost of the pressed pellet method make it the more practical choice.
Ultimately, selecting and mastering a consistent sample preparation protocol is the most critical investment you can make in achieving reliable XRF analysis.
Summary Table:
| Method | Key Process | Best For | Key Equipment |
|---|---|---|---|
| Fusion | Dissolving sample in molten flux to create a glass disk | Maximum accuracy, complex/unknown materials | Fusion furnace, platinum crucible |
| Pressed Pellet | Compressing fine powder into a solid pellet | High-throughput, quality control of consistent materials | Hydraulic press, grinder |
Achieve reliable, accurate results for your lab. The right sample preparation is the foundation of successful XRF analysis. KINTEK specializes in the lab equipment and consumables you need, from hydraulic presses for pellet preparation to fusion furnaces and platinum labware for the ultimate in accuracy. Contact our experts today to discuss your specific application and ensure your sample prep method delivers the precision you require.
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
People Also Ask
- How is a laboratory hydraulic press used for LLZTO pellets? Achieve 93% Density in Solid-State Battery Research
- What is KBr disc method? A Complete Guide to IR Spectroscopy Sample Prep
- Why is a hydraulic pellet press used for FTIR? Transform Nanofillers into Clear Data
- What role does a laboratory hydraulic press play in preparing solid model materials? Standardize for Precise Data.
- How do laboratory hydraulic presses facilitate biomass pelletization? Optimize Biofuel Density and Prevent Slagging



















