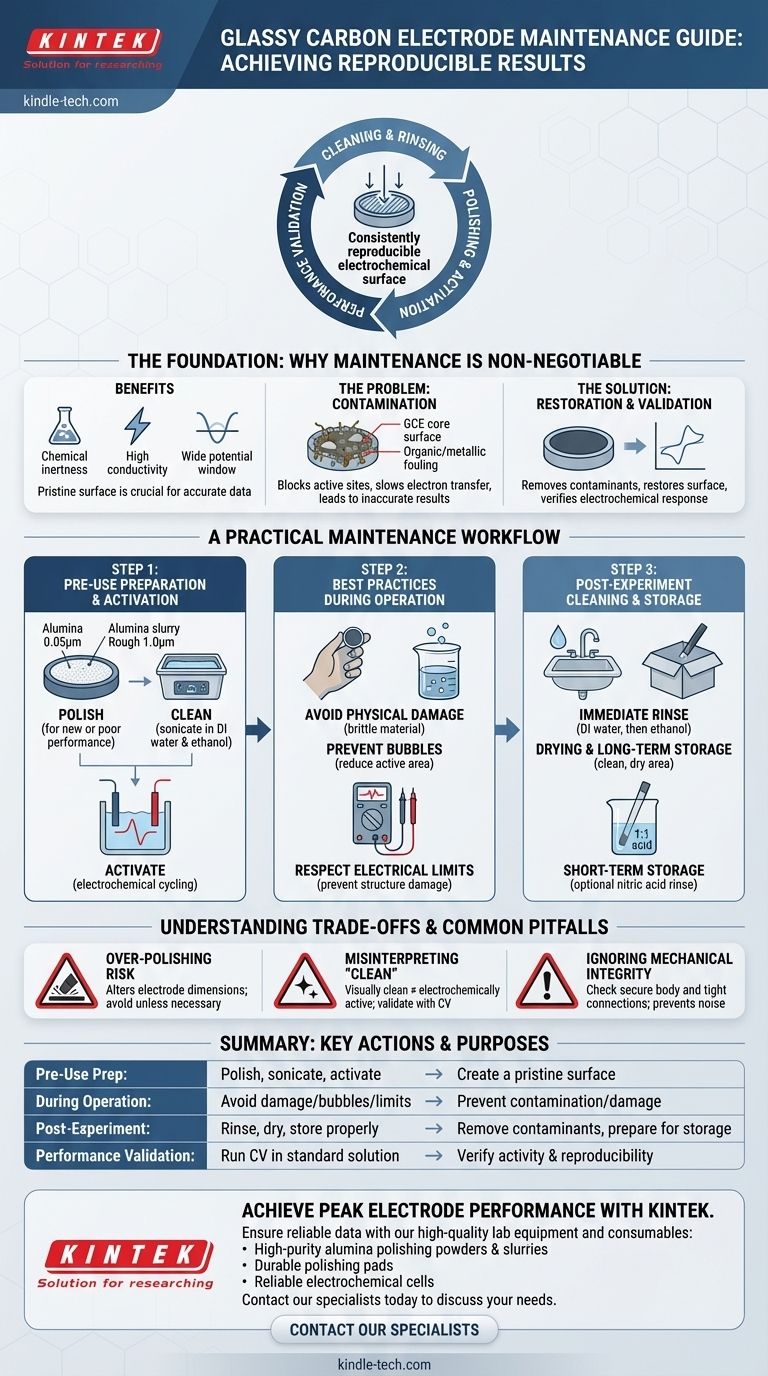
Proper maintenance of a glassy carbon electrode (GCE) is a systematic cycle of cleaning, polishing, and performance validation. After each experiment, the electrode should be rinsed with deionized water and ethanol. For deeper cleaning or to restore performance, it requires polishing with an alumina slurry, followed by chemical or electrochemical activation to ensure its surface is ready for accurate measurements.
The core goal of GCE maintenance extends beyond simple cleaning. It is about creating a consistently reproducible electrochemical surface, which is the foundational requirement for generating reliable and trustworthy experimental data.

The Foundation: Why Maintenance is Non-Negotiable
A glassy carbon electrode is valued for its chemical inertness, high conductivity, and wide potential window. However, these benefits are only realized when its surface is pristine.
The Problem of Surface Contamination
The GCE surface is highly susceptible to contamination from organic compounds, metallic species, or reaction byproducts. This fouling, often invisible, can block active sites, slow down electron transfer, and lead to inaccurate and non-reproducible results.
Restoring the Electrochemical Surface
The primary objective of maintenance is to remove contaminants and restore a smooth, electrochemically active surface. This involves both physical removal of material (polishing) and chemical or electrochemical conditioning (activation).
Validating Electrode Performance
You cannot assume an electrode is performing well just because it looks clean. A quick diagnostic test, such as running a cyclic voltammogram (CV) in a standard potassium ferricyanide solution, is the best way to verify that the electrode's electrochemical response is fast and predictable.
A Practical Maintenance Workflow
A consistent protocol is the key to reproducible results. This workflow should be integrated into your experimental routine, covering preparation, use, and storage.
Step 1: Pre-Use Preparation and Activation
Before a critical experiment, the electrode must be properly prepared.
- Polishing: If the electrode is new, has been stored for a long time, or shows poor performance, it must be polished. Start by gently polishing the surface on a polishing pad with a fine alumina powder slurry (e.g., 0.05 µm). For severely contaminated or scratched surfaces, a rougher polish (e.g., 1.0 µm) may be needed first, followed by the fine polish.
- Cleaning: After polishing, thoroughly sonicate the electrode in deionized water to remove all alumina particles. This can be followed by sonication in ethanol to remove organic residues.
- Activation: The final step is often electrochemical activation. This can be done by cycling the potential in a suitable electrolyte (e.g., dilute sulfuric acid) to condition the surface and remove any final impurities.
Step 2: Best Practices During Operation
Proper handling during an experiment prevents damage and minimizes contamination.
- Avoid Physical Damage: Glassy carbon is hard but brittle. Avoid dropping the electrode or letting it collide with hard or sharp objects, which can cause scratches or fractures.
- Prevent Bubbles: Ensure no air bubbles adhere to the electrode surface during measurements, as this will reduce the active surface area and distort results.
- Respect Electrical Limits: Always operate within the specified current and voltage limits for your system to avoid damaging the electrode structure or generating unintended byproducts.
Step 3: Post-Experiment Cleaning and Storage
Immediate cleaning after use prevents contaminants from drying and hardening on the surface.
- Immediate Rinse: As soon as an experiment is complete, rinse the electrode surface thoroughly with deionized water, followed by an ethanol rinse.
- Drying and Long-Term Storage: Allow the electrode to air dry completely. For long-term storage, place it in its original box in a clean, dry, and ventilated area away from moisture and high temperatures.
- Short-Term Storage: For brief periods between frequent experiments, some protocols suggest immersing the electrode tip in a 1:1 nitric acid solution. It must be thoroughly rinsed with deionized water before its next use.
Understanding the Trade-offs and Common Pitfalls
Effective maintenance requires judgment, not just blind adherence to a checklist. Understanding the potential downsides of each step is crucial for an expert.
The Risk of Over-Polishing
Polishing is an abrasive process that physically removes a layer of the electrode surface. Over-polishing can alter the electrode's dimensions over time and is often unnecessary for routine experiments where a simple rinse or electrochemical cleaning is sufficient.
Misinterpreting a "Clean" Appearance
A visually clean and shiny electrode is not necessarily electrochemically active. Adsorbed, transparent layers of contaminants can render the surface inert. This is why performance validation with a standard redox couple like ferricyanide is the only true test of an electrode's condition.
Ignoring Mechanical Integrity
A perfect surface is useless if the electrical connection is poor. Periodically check that the electrode body is secure in its holder and that all wire connections are tight. A loose connection will introduce noise and resistance, compromising your entire measurement.
How to Apply This to Your Protocol
Your maintenance strategy should align with your experimental goals.
- If your primary focus is routine, high-throughput analysis: A consistent post-experiment rinse with DI water and ethanol, followed by periodic polishing when performance degrades, is a practical approach.
- If your primary focus is sensitive trace analysis or sensor development: A rigorous pre-treatment protocol, including fine polishing and electrochemical activation before every critical experiment, is essential for achieving the lowest detection limits and highest reproducibility.
- If your primary focus is maximizing electrode lifespan: Prioritize careful handling to prevent scratches, adhere to proper storage conditions, and always operate within the specified potential and current limits.
By mastering these procedures, you transform the electrode from a potential source of error into a reliable and controlled component of your electrochemical system.
Summary Table:
| Maintenance Step | Key Action | Purpose |
|---|---|---|
| Pre-Use Preparation | Polish with alumina slurry, sonicate, electrochemically activate | Create a pristine, reproducible surface |
| During Operation | Avoid physical damage, bubbles, and electrical limits | Prevent contamination and physical damage |
| Post-Experiment | Rinse with DI water/ethanol, dry, store properly | Remove contaminants and prepare for storage |
| Performance Validation | Run CV in a standard solution (e.g., ferricyanide) | Verify electrochemical activity and reproducibility |
Achieve Peak Electrode Performance with KINTEK
Ensuring your glassy carbon electrode delivers reliable data is critical for your research. KINTEK specializes in high-quality lab equipment and consumables, including the precise tools needed for effective electrode maintenance.
We provide:
- High-purity alumina polishing powders and slurries.
- Durable polishing pads and accessories.
- Reliable electrochemical cells and accessories.
Let our expertise support your laboratory's success. Contact our specialists today to discuss your specific electrode maintenance needs and discover the right solutions for your lab.
Visual Guide
Related Products
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- Multi-zone Laboratory Tube Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
People Also Ask
- How does an induction graphitization furnace facilitate the transformation of unburned carbon into synthetic graphite?
- Why is graphite the best conductor of heat? Understanding Its Directional Thermal Superiority
- Why does graphite not melt? Unlocking the Secrets of Its Extreme Heat Resistance
- Why can graphite conduct heat? Unlocking Its Anisotropic Thermal Properties
- Why is graphite so hard to melt? The Secret Lies in Its Atomic Structure














