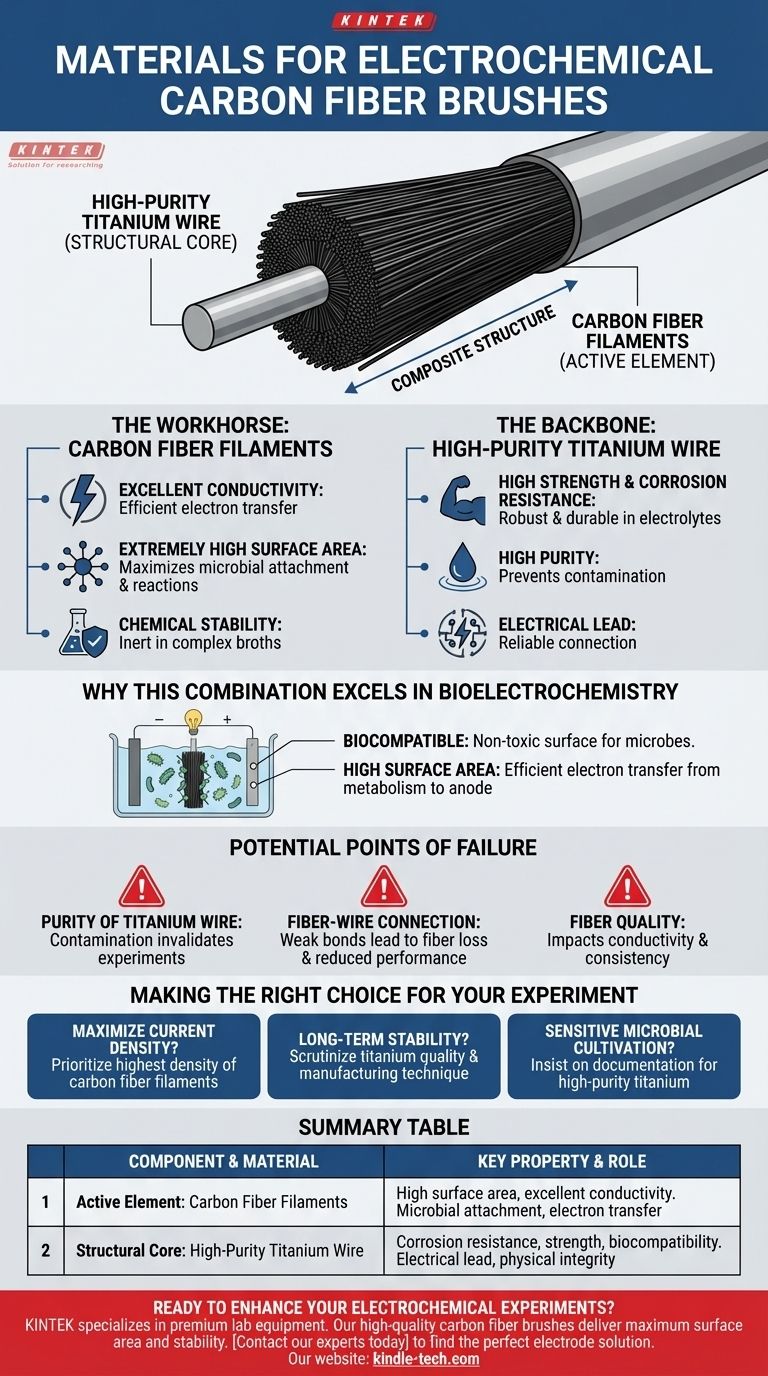
At their core, carbon fiber brushes designed for electrochemical experiments are composite structures. They are manufactured by twisting or binding a massive number of high-quality carbon fiber filaments around a central, structural high-purity titanium wire.
The selection of these two materials is not accidental. The design leverages carbon fiber for its vast, conductive surface area and chemical stability, while using titanium wire as a strong, corrosion-proof backbone to hold the assembly together and provide a reliable electrical connection.

The Role of Each Component
To understand why this specific combination is so effective, we must analyze the function of each material within the electrochemical cell, particularly in applications like microbial fuel cells.
The Workhorse: Carbon Fiber Filaments
The carbon fibers are the active component of the brush. They provide a unique combination of properties essential for electrochemical performance.
First is excellent conductivity. This allows the brush to efficiently collect and transfer electrons, which is its primary function as an electrode.
Second is an extremely high surface area. The thousands of individual filaments create a vast area for microbial colonies to attach and grow, or for electrochemical reactions to occur, dramatically increasing the efficiency and current density of the system.
Finally, carbon fiber offers remarkable chemical stability. It remains inert in the complex chemical broths of microbial experiments, ensuring it does not degrade or release contaminants that could poison the system.
The Backbone: High-Purity Titanium Wire
The titanium wire serves as the structural core and electrical lead for the brush. Its role is just as critical as the carbon fiber.
The primary benefit is high strength and corrosion resistance. Titanium is exceptionally robust and does not rust or corrode, even when submerged for long periods in electrolyte solutions. This ensures the brush's physical integrity and long-term stability.
The emphasis on high purity is crucial. Impurities in a lesser-grade metal could leach into the experimental environment, potentially inhibiting microbial activity or creating unwanted side reactions that compromise the results.
Why This Combination Excels in Bioelectrochemistry
The pairing of carbon fiber and titanium wire makes these brushes an ideal choice for constructing anodes in microbial electrochemical systems.
Biocompatibility and Performance
The materials are biocompatible, meaning they provide a suitable and non-toxic surface for microbes to colonize.
The high surface area of the carbon fibers maximizes the interface between the microbes and the electrode, allowing for efficient electron transfer from the microbial metabolism to the anode. This is why they are a preferred material for microbial cultivation and electrochemical tests.
Potential Points of Failure
While highly effective, the performance of a carbon fiber brush is contingent on its manufacturing quality. Understanding these factors is key to selecting a reliable product.
Purity and Integrity
The purity of the titanium wire is non-negotiable. Any contamination can invalidate an experiment, making verification of material sourcing critical for sensitive applications.
The Fiber-Wire Connection
The physical bond between the carbon fibers and the titanium wire must be secure. A weak connection can lead to fiber loss over time, reducing the brush's surface area and overall performance.
Fiber Quality
The quality of the carbon fiber filaments themselves also matters. Higher-grade fibers offer better conductivity and consistency, leading to more reliable and reproducible experimental results.
Making the Right Choice for Your Experiment
Your specific goal will determine which material property is most important for your application.
- If your primary focus is maximizing current density: Prioritize brushes with the highest density of carbon fiber filaments to achieve the largest possible surface area.
- If your primary focus is long-term stability and reproducibility: Scrutinize the quality of the titanium wire and the manufacturing technique used to bind the fibers, ensuring a robust and durable construction.
- If your primary focus is sensitive microbial cultivation: Insist on documentation confirming the high purity of the titanium wire to prevent any risk of experimental contamination.
Ultimately, the synergy between conductive carbon fibers and a stable titanium core is what makes these brushes a powerful and reliable tool for electrochemical research.
Summary Table:
| Component | Material | Key Property | Role in Experiment |
|---|---|---|---|
| Active Element | Carbon Fiber Filaments | High surface area, excellent conductivity | Microbial attachment, electron transfer |
| Structural Core | High-Purity Titanium Wire | Corrosion resistance, strength, biocompatibility | Electrical lead, physical integrity |
Ready to enhance your electrochemical experiments with reliable, high-performance electrodes?
KINTEK specializes in premium lab equipment and consumables. Our high-quality carbon fiber brushes, built with high-purity titanium cores and superior carbon filaments, are designed to deliver maximum surface area, long-term stability, and reproducible results for your most demanding bioelectrochemical applications.
Contact our experts today to find the perfect electrode solution for your research needs.
Visual Guide
Related Products
- Conductive Carbon Fiber Brush for Static Removal and Cleaning
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Engineering Advanced Fine Ceramics Head Tweezers with Pointed Elbow Zirconia Ceramic Tip
People Also Ask
- Under what conditions should a carbon fiber brush be replaced? Identify Critical Failure to Ensure Performance
- What does the regular maintenance inspection of a carbon fiber brush entail? Ensure Peak Performance and Longevity
- What is the recommended cleaning procedure for a carbon fiber brush after use? Extend Brush Life and Maintain Performance
- What are 3 types of biomass? A Guide to Wood, Waste, and Biofuels for Energy
- What are 3 benefits of biomass energy? Turn Waste into Renewable Power


















