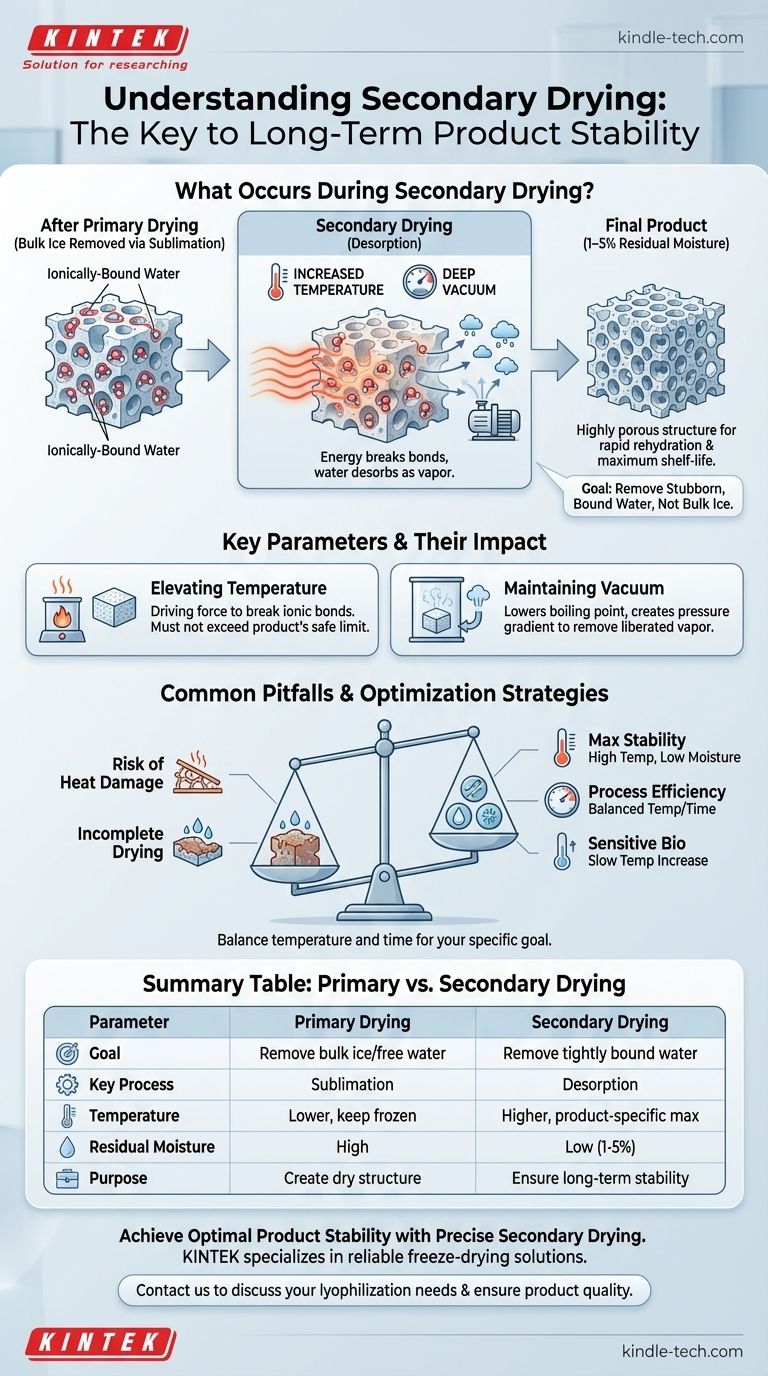
In short, secondary drying removes the final, tightly bound water molecules. This is accomplished by increasing the product's temperature under a deep vacuum, providing enough energy to break the bonds holding this residual moisture to the material. This critical step ensures the long-term stability of the final product.
The core purpose of secondary drying is not to remove bulk ice, but to desorb the stubborn, ionically-bound water that primary drying leaves behind, achieving the lowest possible residual moisture for maximum shelf-life.

The Goal of Secondary Drying: Beyond Free Water
To understand secondary drying, we must first recognize what primary drying accomplishes—and what it leaves behind. The entire process is a carefully controlled sequence to achieve a highly stable, dry product.
Recapping Primary Drying
The first phase, primary drying, removes the bulk of the water from the product. This is done by converting frozen water (ice) directly into vapor through sublimation, all while keeping the product temperature low. This phase removes the "easy" water.
The Challenge of Bound Water
After primary drying, a small but significant amount of water remains. This isn't free ice; it's ionically-bound water, where individual water molecules are attached directly to the product molecules. These bonds are much stronger than the bonds between water molecules in ice.
The Mechanism of Desorption
Secondary drying targets this bound water. By carefully raising the temperature, we provide the necessary thermal energy to break these ionic bonds. This process is called desorption, where the water molecules are released from the product's surface as vapor and removed by the vacuum system.
Key Parameters and Their Impact
The success of secondary drying hinges on the precise control of temperature and pressure to achieve the final desired moisture content without damaging the product.
Elevating the Temperature
The temperature is increased to a level higher than in the primary phase, often to the maximum temperature the product can safely tolerate. This is the driving force that supplies the energy needed to break the bonds holding the last water molecules.
Maintaining the Vacuum
While temperature provides the energy, the deep vacuum is still critical. It lowers the boiling point of water and creates a pressure gradient that efficiently removes the newly liberated water vapor from the drying chamber.
The Result: A Porous, Stable Product
Completing this phase successfully leaves the material with a final residual moisture content typically between 1% and 5%. The removal of both free and bound water creates a highly porous structure, which allows for rapid rehydration when the product is ready for use.
Common Pitfalls to Avoid
While essential, secondary drying is a delicate balance. Incorrectly managing this phase can compromise the entire freeze-drying cycle and ruin the final product.
Risk of Product Degradation
The primary risk is heat damage. If the temperature is raised too high or too quickly, it can cause the product's structure to collapse or, in the case of sensitive biologics, cause denaturation. This damage is irreversible.
Incomplete Drying
Conversely, not providing enough heat or time will result in incomplete removal of bound water. High residual moisture significantly reduces the product's shelf-life and can lead to degradation over time.
Making the Right Choice for Your Goal
Optimizing your secondary drying phase depends entirely on the nature of your product and your ultimate stability requirements.
- If your primary focus is maximum long-term stability: Aim for the lowest possible residual moisture by using the highest temperature your product can tolerate without damage.
- If your primary focus is process efficiency: Carefully balance the temperature ramp rate and hold time to minimize the cycle duration while still hitting your target moisture level.
- If your primary focus is preserving a sensitive biological product: Employ a more conservative and slower temperature increase to ensure the product's molecular structure remains completely intact.
Ultimately, mastering secondary drying is key to producing a stable, effective, and long-lasting lyophilized product.
Summary Table:
| Parameter | Primary Drying | Secondary Drying |
|---|---|---|
| Goal | Remove bulk ice (free water) via sublimation | Remove tightly bound water via desorption |
| Key Process | Sublimation | Desorption |
| Temperature | Lower, to keep product frozen | Higher, product-specific maximum safe temperature |
| Residual Moisture | High (after primary drying) | Low (1-5% final target) |
| Purpose | Create a dry structure | Ensure long-term stability and shelf-life |
Achieve optimal product stability with precise secondary drying.
KINTEK specializes in laboratory freeze-drying equipment and consumables, providing the reliable temperature and vacuum control essential for successfully completing the secondary drying phase. Whether your goal is maximum shelf-life, process efficiency, or preserving sensitive biologics, our solutions are designed to help you achieve the lowest possible residual moisture content for a stable, long-lasting product.
Contact us today to discuss how we can support your specific lyophilization needs and ensure your final product's quality. Get in touch via our contact form to speak with an expert.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- Why is a freeze dryer preferred over thermal drying for Fe-ZTA cermets? Ensure Pure, Homogeneous Slurry Processing
- What is the purpose of an evaporator? The Key Component That Creates Cooling
- Why is a laboratory freeze-drying system essential for fermentation biomass? Preserve Sample Integrity for Analysis
- Why is a laboratory vacuum freeze dryer essential for plant extracts? Preserve Bioactivity & Structure
- What is the function of Freeze-thaw Equipment in Au-(PNiPAAm/PVA) hydrogel? Achieve High-Speed Photothermal Actuation



















