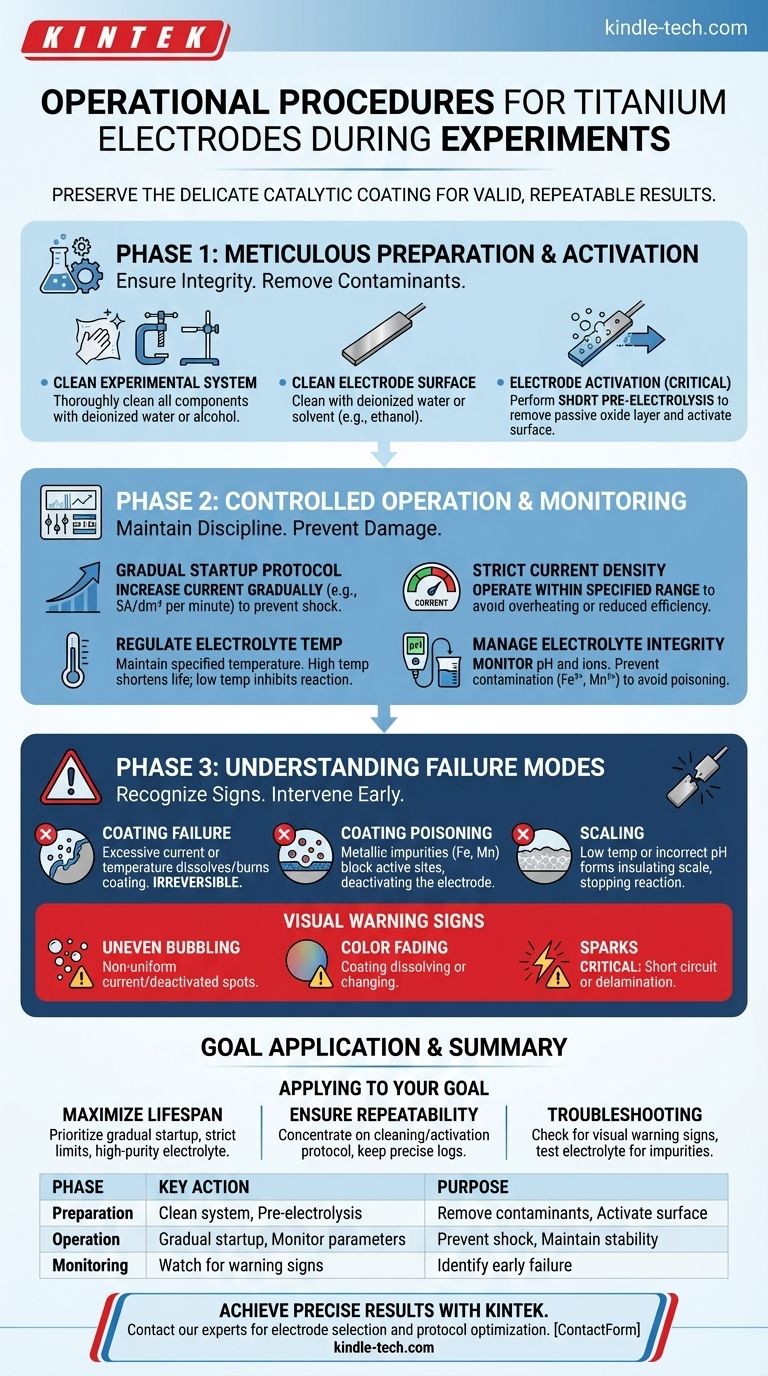
To properly operate a titanium electrode, you must follow a strict protocol encompassing preparation, controlled startup, and in-experiment monitoring. Key steps include meticulously cleaning the electrode and cell, activating the surface via pre-electrolysis, gradually increasing the current to avoid shock, and maintaining the electrolyte's temperature, pH, and purity within the coating's specified limits throughout the procedure.
The core challenge is not just running the experiment, but preserving the delicate catalytic coating on the titanium substrate. Success depends on creating a stable, clean, and controlled electrochemical environment to prevent irreversible damage and ensure valid, repeatable results.

Phase 1: Meticulous Preparation and Activation
Before power is ever applied, the integrity of your experiment is determined by preparation. Contaminants are the primary source of failed experiments and electrode damage.
Cleaning the Experimental System
First, thoroughly clean all components that will contact the electrolyte. This includes electrode clamps, cell fixtures, and support stands.
Wipe these surfaces with deionized water or alcohol to remove any residual electrolyte, grease, or other impurities that could contaminate your system.
Cleaning the Electrode Surface
The electrode itself must be pristine. Clean the titanium electrode surface with deionized water or a suitable solvent like ethanol to remove any organic residues or dust particles.
The Critical Step: Electrode Activation
A clean surface is not enough; it must be electrochemically active. Perform a short period of pre-electrolysis in the electrolyte solution before your main experiment begins.
This crucial step helps remove the passive, non-conductive oxide layer that naturally forms on titanium, ensuring a uniform and active surface for your reaction.
Phase 2: Controlled Operation and Monitoring
Once the system is prepared, operational discipline is paramount. Abrupt changes or deviations from specified parameters are the fastest way to ruin an electrode.
The Gradual Startup Protocol
Never apply full power at once. Increase the current gradually to prevent thermal and electrical shock to the electrode's coating.
A controlled ramp-up, for example at a rate of 5A/dm² per minute, prevents delamination of the coating and ensures a stable start.
Maintaining Strict Current Density
Operate the electrode strictly within the specified current range for its coating.
Exceeding this limit causes overheating, rapid coating dissolution, and permanent failure. Operating too far below the limit can significantly reduce process efficiency.
Regulating Electrolyte Temperature
Maintain the electrolyte temperature as required by the process specifications.
High temperatures accelerate the dissolution of the electrode's catalytic coating, shortening its life. Excessively low temperatures can inhibit the reaction rate or cause unwanted scaling on the electrode surface.
Managing Electrolyte Integrity
The electrolyte is a dynamic environment that must be managed. Regularly monitor its pH and key ion concentrations.
Furthermore, actively prevent contamination from impurities. Ions like iron (Fe³⁺) and manganese (Mn²⁺) are particularly harmful and can "poison" the coating, rendering it inactive.
Understanding Common Failure Modes
Recognizing the signs of failure allows you to intervene before the electrode is permanently damaged. Your primary role during the experiment is to be an active observer.
The Risk of Coating Failure
Running the electrode with excessive current or at too high a temperature will cause the catalytic coating to literally dissolve or burn off the titanium substrate. This damage is irreversible.
The Problem of "Coating Poisoning"
Certain metallic impurities, even at low concentrations, can deposit onto the active sites of the electrode. This process, known as poisoning, blocks the sites needed for your reaction and deactivates the electrode.
The Consequence of Scaling
Operating at low temperatures or with incorrect electrolyte pH can cause salts or other compounds to precipitate out of the solution and form a hard layer, or scale, on the electrode. This scale insulates the electrode, stopping the reaction.
Interpreting Visual Warning Signs
Constant observation is your best diagnostic tool. If you see any of the following, stop the experiment immediately:
- Uneven bubbling: Indicates non-uniform current distribution or deactivated spots.
- Color fading: Suggests the catalytic coating is dissolving or changing composition.
- Sparks: A critical sign of a short circuit or coating delamination.
How to Apply This to Your Goal
Your operational focus will shift slightly depending on your primary objective for the experiment.
- If your primary focus is maximizing electrode lifespan: Prioritize a gradual startup, strict adherence to current and temperature limits, and maintaining a high-purity electrolyte.
- If your primary focus is ensuring experimental repeatability: Concentrate on the meticulous cleaning and activation protocol, and keep a precise log of all operating parameters (current, voltage, temperature, pH).
- If your primary focus is troubleshooting a failing experiment: Immediately check for visual warning signs and test the electrolyte for common impurities like iron and manganese.
By treating the electrode as a precision instrument rather than a simple component, you ensure the validity of your work and the longevity of your equipment.
Summary Table:
| Phase | Key Action | Purpose |
|---|---|---|
| Preparation | Clean system & electrode; Pre-electrolysis | Remove contaminants; Activate surface |
| Operation | Gradual startup; Monitor current, temperature, pH | Prevent shock; Maintain coating stability |
| Monitoring | Watch for uneven bubbling, color fading, sparks | Identify early failure signs |
Achieve precise and reliable electrochemical results with KINTEK. Proper electrode handling is critical for valid data and long-lasting equipment. Our range of high-quality lab electrodes and consumables is designed for demanding applications. Let our experts help you select the right tools and optimize your protocols. Contact our team today to discuss your specific laboratory needs and ensure the success of your experiments.
Visual Guide
Related Products
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Gold Disc Electrode
People Also Ask
- What is the application of RRDE? Unlock Quantitative Catalyst and Reaction Insights
- What is the common role of a platinum disk electrode? A Guide to Its Primary Use as a Working Electrode
- How should a platinum wire/rod electrode be cleaned before use? A Guide to Reliable Electrochemical Data
- What is the difference between RDE and RRDE? Unlock Advanced Electrochemical Reaction Analysis
- What is the rotating ring disk electrode method? Unlock Real-Time Reaction Analysis



















