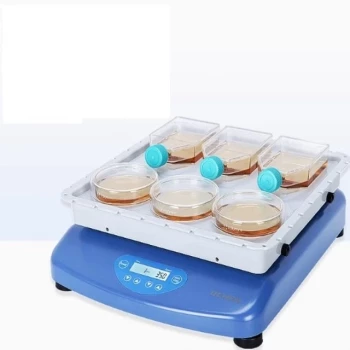
A reciprocating linear shaker acts as the mechanical engine for consistency during the formaldehyde extraction process. It creates a constant kinetic environment that forces leather samples to mix thoroughly with surfactant solutions, such as sodium dodecyl sulfate. This controlled motion ensures the vigorous mass transfer necessary to release formaldehyde trapped within the leather's resin matrix.
By maintaining a specific oscillation frequency, the shaker guarantees that the solvent makes total contact with the leather powder. This mechanical precision transforms a variable manual process into a highly efficient, reproducible method for reliable detection.
The Mechanics of Efficient Extraction
Creating a Constant Kinetic Environment
Reliable chemical analysis requires a stable environment. A reciprocating linear shaker replaces variable manual mixing with a constant kinetic input.
This ensures that every sample receives the exact same amount of mechanical energy. This consistency is vital for comparing results across different batches of leather.
Ensuring Vigorous Mass Transfer
To extract formaldehyde, the solvent must penetrate the solid material. The shaker generates vigorous mass transfer by rapidly oscillating the mixture.
This movement breaks down the boundary layers between the solid leather powder and the liquid solvent. It forces the chemicals to interact faster and more deeply than static soaking allows.
Maximizing Surface Contact
Leather powder can be resistant to wetting. The reciprocating action ensures thorough contact between every particle of leather and the solution.
This prevents dry pockets or clumping that could hide formaldehyde from the extraction process.
The Chemical Interaction
Optimizing Surfactant Performance
The primary reference highlights the use of surfactant solutions like sodium dodecyl sulfate (SDS). The shaker is essential for distributing this surfactant evenly.
Proper agitation allows the SDS to effectively reduce surface tension and penetrate the leather structure. Without this mechanical aid, the surfactant cannot perform at peak efficiency.
Dissolving the Resin Matrix
Formaldehyde is often bound within the resin matrix of the leather. The goal of the process is the completeness of dissolution.
The shaker's specific oscillation frequency provides the physical force needed to liberate these bound chemicals. This ensures that the formaldehyde detected represents the total amount present, not just the surface residue.
Understanding the Variables
The Importance of Oscillation Frequency
The efficiency of the extraction is directly tied to the oscillation frequency.
If the frequency is too low, the mass transfer will be weak, leading to incomplete extraction. The shaker allows you to lock in a specific frequency to avoid this pitfall.
Avoiding False Negatives
The biggest risk in this process is under-extraction. If the mixing is not vigorous enough, formaldehyde remains trapped in the leather.
This leads to lower detection results, falsely suggesting the leather is safe when it may not be. The shaker mitigates this risk by driving the process to completion.
Ensuring Reliable Detection Results
To maximize the accuracy of your formaldehyde analysis, you must align your equipment settings with your analytical goals.
- If your primary focus is Consistency: Ensure your shaker allows you to lock in a specific oscillation frequency to eliminate batch-to-batch variability.
- If your primary focus is Sensitivity: Verify that the kinetic environment is vigorous enough to fully wet the leather powder with the sodium dodecyl sulfate solution.
Precision in extraction is the only path to precision in detection.
Summary Table:
| Feature | Role in Formaldehyde Extraction | Impact on Results |
|---|---|---|
| Reciprocating Motion | Facilitates vigorous mass transfer between leather and solvent | Ensures complete dissolution of resin matrix |
| Oscillation Frequency | Provides constant kinetic energy for uniform mixing | Eliminates batch-to-batch variability |
| Mechanical Agitation | Maximizes contact between surfactant (SDS) and leather powder | Prevents false negatives from incomplete extraction |
| Automated Precision | Replaces manual shaking with repeatable mechanical input | Guarantees reliable, reproducible detection results |
Elevate Your Laboratory Precision with KINTEK
Don't compromise your chemical analysis with inconsistent results. KINTEK specializes in high-performance laboratory equipment designed to meet the rigorous demands of material testing. Whether you are performing formaldehyde extraction with our precision shakers and homogenizers or conducting advanced material research using our high-temperature furnaces, hydraulic presses, and crushing systems, we provide the tools for success.
From leather testing to battery research and dental ceramics, our comprehensive portfolio—including PTFE consumables, ULT freezers, and electrolytic cells—is engineered for reliability.
Ready to ensure the accuracy of your detection results? Contact KINTEK today to discover how our expert solutions can streamline your extraction processes and empower your lab's productivity!
References
- Moêz Smiri, Soumaya Elarbaoui. Removal of Chromium (Cr) and Formaldehyde[CH<sub>2</sub>O (H−CHO)] from Leather Tannery EffluentsUsing Electrocoagulation Treatment Process. DOI: 10.15244/pjoes/157494
This article is also based on technical information from Kintek Solution Knowledge Base .
Related Products
- Laboratory Multifunctional Small Speed-Adjustable Horizontal Mechanical Shaker for Lab
- Shaking Incubators for Diverse Laboratory Applications
- Laboratory Oscillating Orbital Shaker
- Vibratory Sieve Shaker Machine Dry Three-Dimensional Vibrating Sieve
- Custom PTFE Teflon Parts Manufacturer PTFE Beaker and Lids
People Also Ask
- How do you maintain a sieve shaker? Ensure Accurate Particle Size Analysis Every Time
- What are types of sieve shakers? Choose the Right Agitation for Accurate Particle Analysis
- What is the efficiency of a vibrating screen? Master the Balance Between Recovery, Purity & Throughput
- How do high-precision sieving systems benefit zeolite preparation? Maximize Adsorption for Wastewater Treatment
- How does a laboratory shaker or extractor function during 133Ba adsorption? Optimize Your Kinetic Evaluation
- What is the role of a laboratory shaker in PHA research? Accelerate Extremophile Screening & Bioplastic Development
- What is the machine used for sieve? Automate Your Particle Analysis with a Sieve Shaker
- What is the principle of vibrating sieve? Achieve Precise Particle Separation with Mechanical Vibration