In short, gas flow is the primary mechanism for actively controlling the chemical environment inside a furnace. It is not a passive element but a critical variable used to protect furnace components, remove unwanted byproducts, and ensure the final quality and integrity of the parts being processed. The rate, composition, and direction of this flow are all precisely managed to create a specific, consistent atmosphere required for a given metallurgical process.
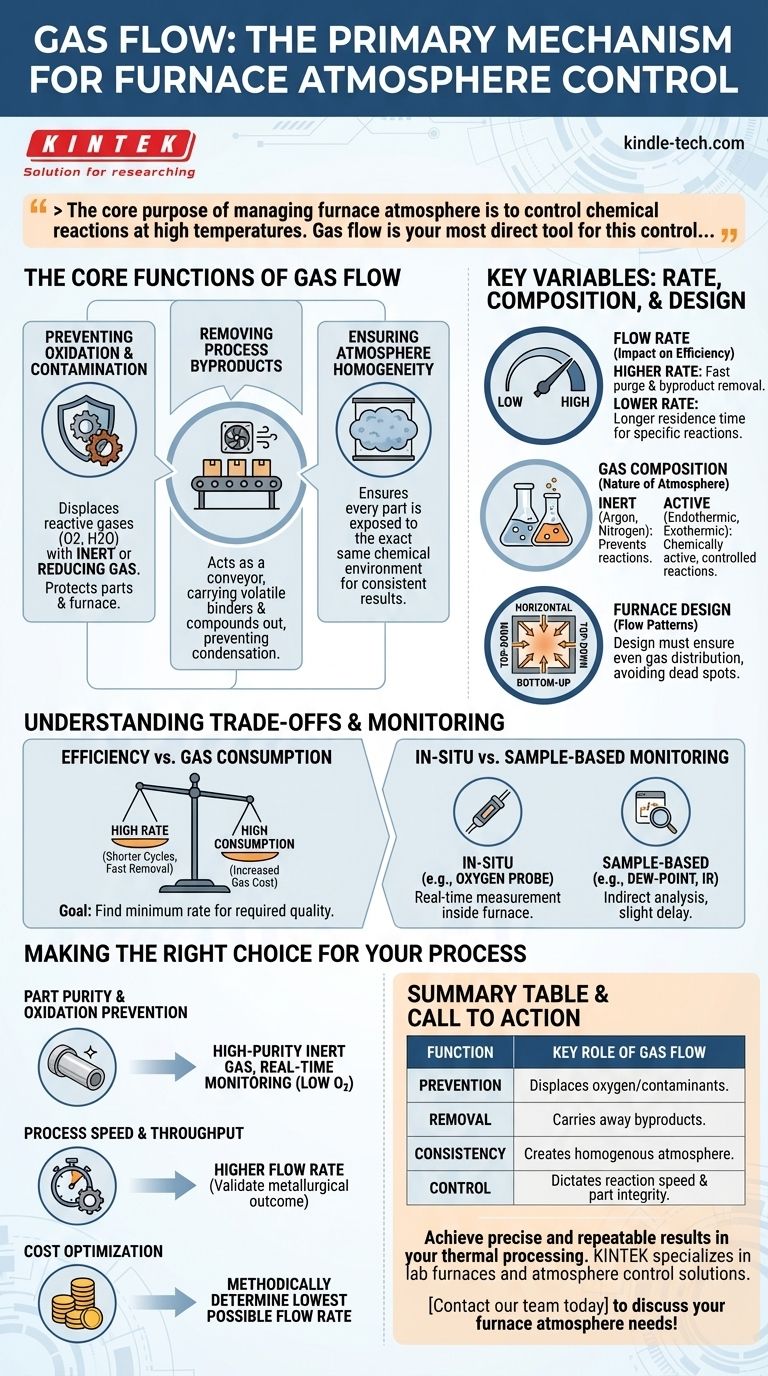
The core purpose of managing furnace atmosphere is to control chemical reactions at high temperatures. Gas flow is your most direct tool for this control, dictating everything from preventing oxidation to influencing reaction speed and efficiency.

The Core Functions of Gas Flow
Gas flow serves several distinct but interconnected purposes within a furnace. Understanding these functions is key to diagnosing issues and optimizing any heat treatment cycle.
Preventing Oxidation and Contamination
The most fundamental role of gas flow is to create a controlled atmosphere that prevents unwanted reactions, primarily oxidation.
By constantly flowing an inert gas (like argon or nitrogen) or a reducing gas through the chamber, you physically displace reactive gases like oxygen and water vapor. This protects both the parts and the internal furnace components from damage.
Removing Process Byproducts
Many thermal processes, such as debinding, release volatile compounds from the parts being treated.
A steady gas flow acts as a conveyor, carrying these binders and other byproducts out of the furnace. This prevents them from re-condensing on cooler surfaces or interfering with the primary metallurgical process.
Ensuring Atmosphere Homogeneity
Without active flow, a furnace atmosphere can become stratified, with different gas compositions in different areas.
Properly engineered gas flow ensures the atmosphere is homogenous, meaning every part within the furnace load is exposed to the exact same chemical environment, leading to consistent and predictable results.
Key Variables: Rate, Composition, and Design
Effective atmosphere control depends on carefully balancing several factors. The "right" approach is determined entirely by the process requirements.
The Impact of Flow Rate
The speed at which gas moves through the furnace has a direct effect on the process.
A higher flow rate is more effective at purging the chamber and removing byproducts quickly, which can enhance process efficiency. However, a lower flow rate may be required for certain reactions where longer residence time is needed to achieve the desired outcome or a higher yield.
The Importance of Gas Composition
The type of gas used defines the nature of the atmosphere. Atmospheres can be inert, preventing any reaction, or they can be chemically active.
For example, endothermic or exothermic atmospheres are created with specific gas mixtures to achieve a desired carbon potential on the surface of a steel part. Adding a small amount of a reactive gas like oxygen can increase a reaction's rate, but an excess can easily cause undesirable oxidation.
The Influence of Furnace Design
The physical construction of the furnace dictates how gas moves.
Flow patterns can be horizontal, top-down, or bottom-up. The design must ensure that the gas reaches all parts of the workload evenly, avoiding dead spots where the atmosphere could stagnate.
Understanding the Trade-offs and Monitoring
Achieving the perfect atmosphere requires balancing competing priorities and using accurate measurement tools to verify the environment.
Efficiency vs. Gas Consumption
A high gas flow rate can shorten cycle times by removing byproducts faster, but it also leads to significantly higher consumption of expensive gases like argon. The goal is to find the minimum flow rate that achieves the required part quality.
In-Situ vs. Sample-Based Monitoring
Verifying the atmosphere is critical. An oxygen probe is an in-situ device that measures the atmosphere directly inside the furnace in real time.
In contrast, dew-point analyzers and infrared analyzers are indirect techniques that rely on pulling a gas sample from the furnace for analysis. While effective, this method introduces a slight delay and may not perfectly represent the dynamic conditions inside the chamber.
Making the Right Choice for Your Process
Your approach to gas flow should be directly tied to your primary process objective.
- If your primary focus is part purity and preventing oxidation: Use a consistent flow of high-purity inert gas and implement real-time monitoring to ensure oxygen levels remain exceptionally low.
- If your primary focus is process speed and throughput: A higher flow rate can be beneficial for rapidly purging byproducts like binders, but you must validate it doesn't negatively impact the desired metallurgical outcome.
- If your primary focus is cost optimization: Methodically determine the lowest possible flow rate that still meets all quality specifications to minimize gas consumption over time.
Ultimately, mastering gas flow is fundamental to achieving repeatable, high-quality results in any thermal processing environment.
Summary Table:
| Function | Key Role of Gas Flow |
|---|---|
| Prevention | Displaces oxygen/contaminants to prevent oxidation. |
| Removal | Carries away process byproducts like binders. |
| Consistency | Creates a homogenous atmosphere for uniform results. |
| Control | Dictates reaction speed and final part integrity. |
Achieve precise and repeatable results in your thermal processing. The right gas flow strategy is critical for protecting your materials and optimizing furnace performance. KINTEK specializes in lab furnaces and atmosphere control solutions. Our experts can help you select the ideal equipment and consumables for your specific application, ensuring quality and efficiency. Contact our team today to discuss your furnace atmosphere needs!
Visual Guide
Related Products
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What is the use of hydrogen in furnace? A Key to Oxygen-Free High-Temperature Processing
- Why are controlled atmosphere furnaces required for NCD coating modification? Unlock Superior Biocompatibility.
- What is the role of a program-controlled carbonization furnace in the preparation of lignin-based carbon fiber? Explained
- What is the primary function of an industrial atmosphere sintering furnace? Achieve Dense, High-Strength Components
- What role does a high-temperature atmosphere furnace play in evaluating oxidation resistance? Optimize Coating Analysis
- Why Use Inert Gas in High-Temperature Reduction Furnaces? Master High-Performance Silicon Carbide Powder Production
- What is controlled atmosphere furnace? Precision Heating Without Oxidation for Superior Materials
- How does an atmosphere furnace influence copper hollow fiber membranes? Stabilize Pore Structure during Sintering



















