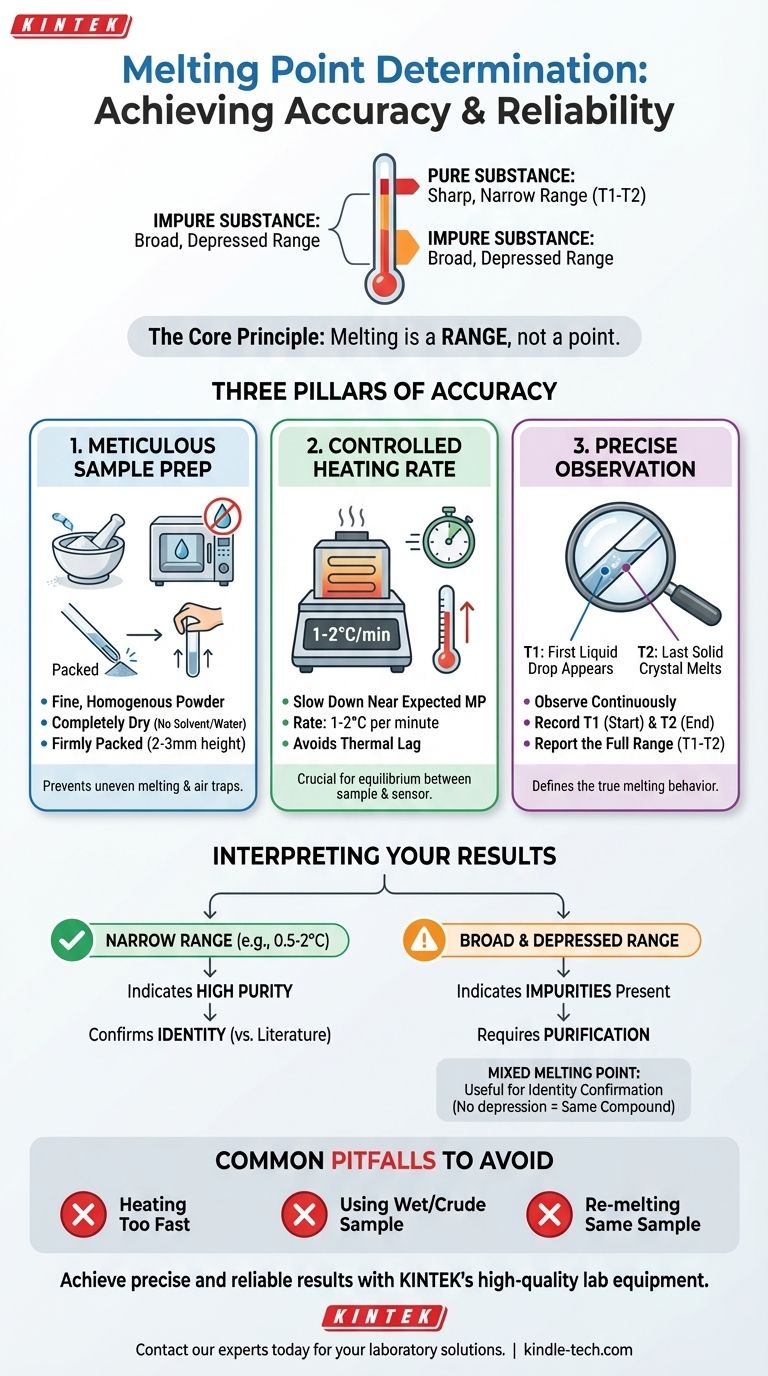
In practice, accurate melting point determination hinges on three factors: meticulous sample preparation, a slow and controlled heating rate, and precise observation of the entire melting range. The technique is a fundamental tool in chemistry, used primarily to confirm the identity of a compound and to assess its purity. A properly executed measurement provides clear, reproducible data.
The core principle to understand is that melting point is not a single temperature but a range. For a pure substance, this range is narrow and sharp. For an impure substance, the range becomes broad and depressed, meaning it begins melting at a lower temperature and over a wider span.

The Principle: What Happens During Melting?
A Transition of States
Melting is the physical process where a substance transitions from a highly ordered solid crystalline lattice to a disordered liquid state. This requires energy, in the form of heat, to overcome the intermolecular forces holding the molecules in their fixed positions.
The Definition of a Melting Range
The melting point is officially recorded as a range of temperatures. The first temperature (T1) is the point at which the first drop of liquid appears among the solid crystals. The second temperature (T2) is the point at which the last crystal of solid melts into liquid.
Key Factors for an Accurate Measurement
To obtain a reliable melting range, you must control several key variables. Each step is designed to ensure that the temperature you read on the thermometer थर्मल is the true temperature of the sample.
Sample Preparation is Critical
The sample must be a fine, homogenous powder. Large crystals melt unevenly and trap air, leading to poor heat transfer and an inaccurate, broad range.
The sample must also be completely dry. Any residual solvent, including water from the air, will act as an impurity and depress the melting point.
Finally, the sample should be packed यूरोप firmly into a capillary tube to a height of no more than 2-3 mm. Too much sample will cause a significant temperature difference between the top and bottom, artificially broadening the melting range.
The Importance of a Slow Heating Rate
This is the most common source of error. Near the expected melting point, the heating rate must be slowed to 1-2 °C per minute.
If you heat the sample too quickly, the temperature of the heating block will rise faster than the heat can transfer to the sample and the thermometer. This "thermal lag" results in the observed melting range being significantly higher than the true value.
Accurate Temperature Observation
You must observe the sample यूरोप continuously as it approaches the melting point. Record the temperature (T1) the instant you see the first tiny droplet of liquid form.
Continue to watch both the sample and the thermometer. Record the second temperature (T2) at the exact moment the last solid particle disappears. The final reported value is always this range: T1 - T2.
Interpreting Your Results: Purity and Identity
The melting range is a powerful diagnostic tool. Its width and position जर्मनी tell a story about your compound.
The Signature of a Pure Compound
A pure, crystalline organic compound will have a sharp, narrow melting range, typically spanning only 0.5 °C to 2 °C. Its observed range will also be in close agreement with the established literature value for that substance.
The Effect of Impurities (Melting Point Depression)
Impurities disrupt the uniform structure of the crystalline lattice. This makes the lattice less stable and requires less energy (a lower temperature) to break it apart.
As a result, an impure substance will exhibit a melting point depression. Its melting range will be both lower and broader than that of the pure compound. The more impurity present, the greater the depression and the broader the range.
Using Mixed Melting Point for Identification
This technique is used to confirm the identity of an unknown substance (A) when you suspect it is a known compound (X).
You prepare a sample containing an intimate mixture of A and X, typically in a 1:1 ratio. If A and X are the same compound, the melting point of the mixture will be sharp and identical to that of pure X. If A and X are different, A will act as an impurity to X (and vice-versa), causing a significant melting point depression and a broad range.
Common Pitfalls and How to Avoid Them
Even experienced chemists can make mistakes. Being aware of these common errors is the first step to preventing them.
Heating Too Quickly
This is the cardinal sin of melting point determination. It consistently leads to a melting range that is both artificially high and broad. Always perform a rapid "scout" measurement first to find the approximate range, then perform a second, slow measurement for accuracy.
Using a "Wet" or Unpurified Sample
Never take a melting point of a crude product or a sample that is not completely dry. The results will be misleading and will not reflect the properties of the pure compound.
Re-melting the Same Sample
Never re-use a sample that has already been melted. Many organic compounds decompose<y_bin_172> slightly at their melting point. A second measurement on the same sample will often show a depressed and broad range due to this self-generated impurity. Always use a fresh sample for each trial.
Making the Right Choice for Your Goal
Your approach to melting point determination depends on your objective.
- If your primary focus is to confirm the identity of a substance: Compare your sharp, narrow melting range against the literature value and, if possible, perform a mixed melting point with an authentic sample.
- If your primary focus is to assess the purity of your product: A narrow melting range (e.g., < 2 °C) that is close to the literature value is a strong indicator of high purity. A broad, depressed range signals the need for further purification.
- If your primary focus is to characterize a new, unknown compound: Perform multiple, careful measurements to establish a highly reproducible and narrow melting range, which serves as a key physical constant for your new substance.
Mastering this seemingly simple technique is a cornerstone of sound chemical analysis and characterization.
Summary Table:
| Factor | Key Consideration | Impact on Result |
|---|---|---|
| Sample Preparation | Fine, dry powder, packed firmly in capillary | Ensures sharp, narrow melting range |
| Heating Rate | Slow rate (1-2°C/min) near melting point | Prevents thermal lag and inaccurate high readings |
| Observation | Record first liquid (T1) and last solid (T2) | Defines the true melting range (T1-T2) |
| Interpretation | Narrow range = pure compound; Broad, depressed range = impure | Critical for assessing purity and confirming identity |
Achieve precise and reliable results in your laboratory.
Accurate melting point determination is fundamental for confirming compound identity and assessing purity. KINTEK specializes in providing high-quality lab equipment and consumables designed to support these critical analyses. From reliable melting point apparatus to essential sample preparation tools, our products are crafted to enhance the accuracy and efficiency of your work.
Let us help you master this cornerstone technique. Contact our experts today to find the perfect solutions for your laboratory's specific needs and ensure your chemical analysis is built on a foundation of precision.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the main components of a high-temperature muffle furnace? A Guide to the Core Systems
- What is a muffle furnace in the environment? Achieve Clean, Contaminant-Free Heating
- What is the working principle of a muffle furnace? Achieve Precise, Contamination-Free Heating
- What is the primary characteristic of a muffle furnace? Unlock Pure, Contamination-Free Heating
- What is a muffle furnace used in determination of? Precise Ash Content and Material Composition



















