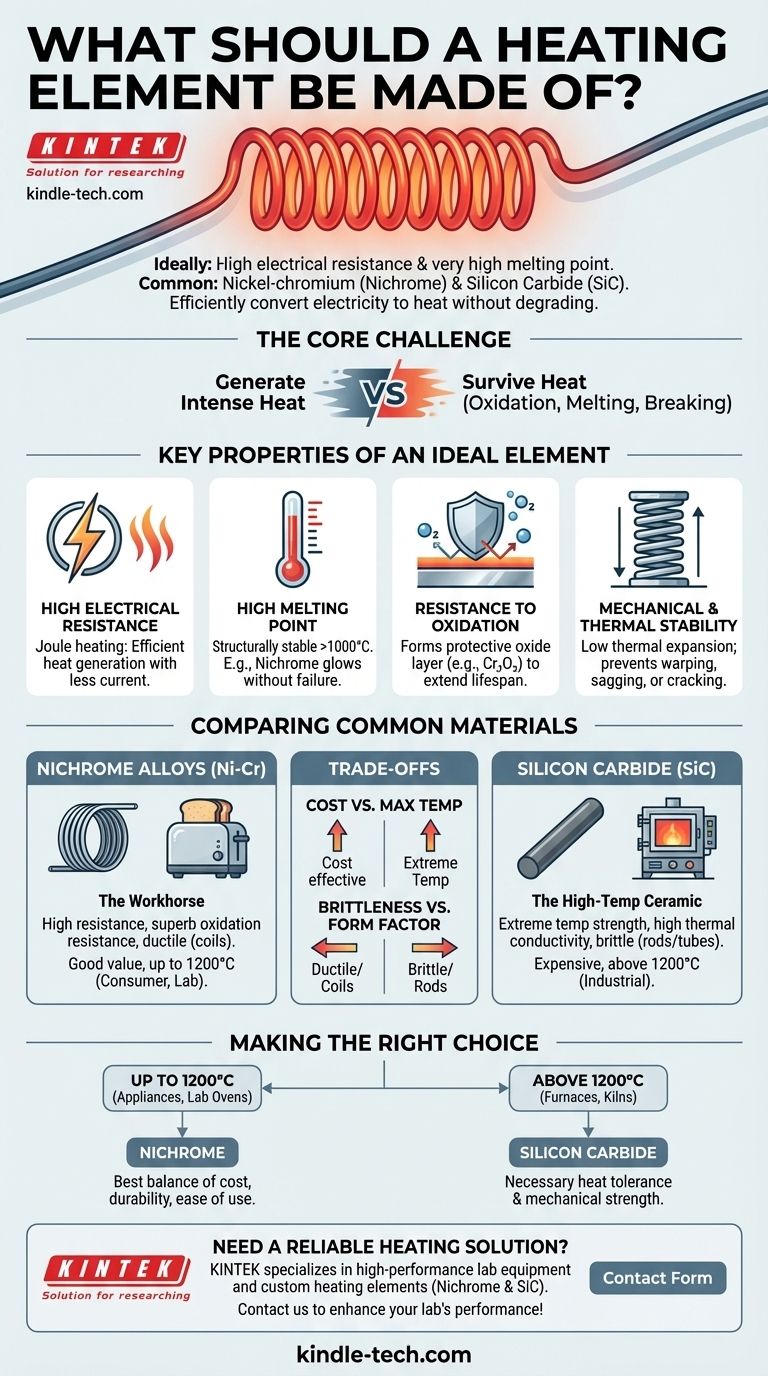
In short, the ideal heating element is made from a material with high electrical resistance and a very high melting point. The most common and effective materials are nickel-chromium alloys (often called Nichrome) for general applications, and ceramics like silicon carbide for extremely high-temperature industrial uses. These materials efficiently convert electricity into heat without quickly degrading or melting.
The core challenge is finding a material that can both generate intense heat through electrical resistance and survive that same heat without oxidizing, melting, or breaking. The choice depends entirely on the target temperature and operating environment.

The Key Properties of an Ideal Heating Element
To understand why certain materials are chosen, we must first define the essential characteristics required to function effectively and reliably as a heater.
High Electrical Resistance
A heating element works by a principle called Joule heating. As electricity flows through a material with resistance, electrical energy is converted directly into thermal energy (heat).
A material with high resistance generates significant heat with less current, making the process efficient and controllable.
High Melting Point
This is a non-negotiable requirement. The material must remain solid and structurally stable far above its intended operating temperature.
Materials like nickel-chromium alloys are selected specifically because they can glow red-hot (reaching temperatures over 1000°C) without losing their integrity.
Resistance to Oxidation
When materials get hot, they react more readily with oxygen in the air, a process called oxidation. For many metals, this is a destructive process akin to accelerated rusting, causing the element to thin and fail.
Ideal heating materials like Nichrome form a stable, protective outer layer of chromium oxide. This layer seals the underlying metal from the air, dramatically extending the element's lifespan.
Mechanical and Thermal Stability
The material must not become overly brittle or soft when hot. It also needs low thermal expansion, as mentioned for silicon carbide.
This stability ensures the element doesn't warp, sag, or crack after repeated cycles of heating and cooling, which would lead to premature failure.
Comparing Common Heating Element Materials
While many materials exist, two categories cover the vast majority of applications, from household appliances to industrial furnaces.
Nickel-Chromium (Nichrome) Alloys
Nichrome is the workhorse of the heating element world. It is an alloy of nickel and chromium and is found in countless devices like toasters, hair dryers, and space heaters.
Its popularity comes from its excellent balance of high resistance, superb oxidation resistance, and relative flexibility, allowing it to be easily formed into coils.
Silicon Carbide (SiC)
Silicon carbide is a ceramic compound used when temperatures must exceed the limits of metallic alloys. It is common in industrial kilns and furnaces.
As a ceramic, it is exceptionally hard and possesses extremely high-temperature strength and thermal conductivity. Unlike Nichrome, it is more brittle and is typically formed into rods or tubes rather than fine coils.
Understanding the Trade-offs
Choosing a material is always an engineering compromise between performance, lifespan, and cost.
Cost vs. Maximum Temperature
Nichrome alloys offer fantastic performance for their cost, making them the default choice for consumer and light commercial applications.
Silicon carbide elements are more expensive but are one of the few practical options for achieving the sustained, extreme temperatures required in heavy industry.
Brittleness vs. Form Factor
Metallic alloys like Nichrome are ductile, meaning they can be drawn into wires and wound into complex coil shapes to fit compact spaces.
Ceramics like silicon carbide are very strong but brittle. This limits their shape, which is why they are typically used in simpler, more robust forms like solid rods.
Making the Right Choice for Your Application
Your final decision should be guided by your specific operational requirements.
- If your primary focus is general-purpose heating up to 1200°C (e.g., appliances, lab ovens): Nickel-chromium alloys offer the best combination of cost, durability, and ease of use.
- If your primary focus is high-temperature industrial processes above 1200°C (e.g., furnaces, kilns): Silicon carbide provides the necessary heat tolerance and mechanical strength that metallic elements cannot.
Ultimately, selecting the right material ensures your heating element is not only effective but also safe and durable for its intended task.
Summary Table:
| Material | Key Properties | Ideal Temperature Range | Common Applications |
|---|---|---|---|
| Nickel-Chromium (Nichrome) Alloys | High resistance, excellent oxidation resistance, ductile | Up to 1200°C | Toasters, lab ovens, space heaters |
| Silicon Carbide (SiC) | Extreme high-temperature strength, high thermal conductivity, brittle | Above 1200°C | Industrial kilns, high-temperature furnaces |
Need a reliable heating solution for your lab or industrial process? KINTEK specializes in high-performance lab equipment and consumables, including custom heating elements designed for precision, durability, and efficiency. Whether you require Nichrome alloys for moderate temperatures or silicon carbide for extreme heat, our experts will help you select the ideal material for your application. Contact us today to discuss your heating needs and enhance your lab's performance!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Glassy Carbon Electrochemical Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
People Also Ask
- How do you check the temperature of a heating element? Choose the Right Tool for Accurate Results
- How long does fiber insulation last? The Truth About Its Real Lifespan & Performance
- Why does my heating element keep going out? Stop the cycle of failure with these expert solutions.
- What is the purpose of using a Pt-Rh thermocouple in magnesium experiments? Ensure Precise Vapor Collection
- What is the most common type of temperature sensor? The Unmatched Versatility of Thermocouples
- What causes heating element failure? Prevent Downtime by Understanding the Degradation Process
- What is the operating principle of a resistance wire heater? Insights into Joule Heating and Precise Thermal Control
- Why are separate thermocouples required for magnesium vacuum sublimation? Ensure Precision & Protect Your Equipment









