At its core, an electrolytic cell uses two primary electrodes: a positively charged anode where oxidation occurs, and a negatively charged cathode where reduction occurs. For more precise analytical work, a three-electrode system is used, consisting of a working electrode, a counter electrode, and a reference electrode.
The type and number of electrodes used in an electrolytic cell are determined by its purpose. A simple two-electrode setup (anode and cathode) is sufficient for driving a chemical reaction, while a three-electrode system is necessary for precisely measuring and controlling it.

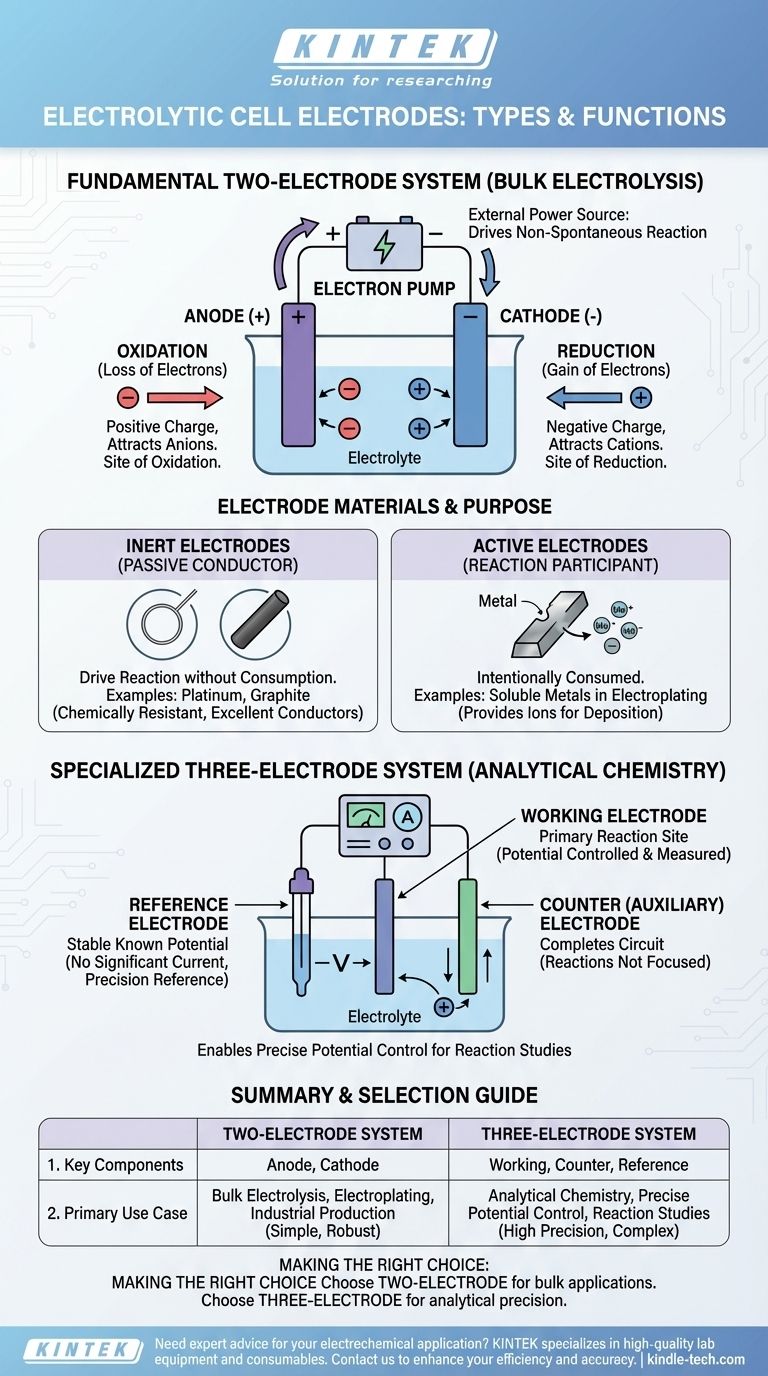
The Fundamental Two-electrode System
Most electrolytic cells, from classroom demonstrations to industrial-scale production, are built on a two-electrode foundation. This system uses an external power source to force a non-spontaneous chemical reaction to occur.
The Anode: Site of Oxidation
The anode is the electrode connected to the positive terminal of the power source. Because of its positive charge, it attracts negatively charged ions (anions) from the electrolyte solution. At the anode's surface, these ions lose electrons in a process called oxidation.
The Cathode: Site of Reduction
Conversely, the cathode is connected to the negative terminal of the power supply. Its negative charge attracts positively charged ions (cations). At the cathode's surface, these ions gain electrons in a process known as reduction.
The Role of the External Power Source
Unlike a battery (a galvanic cell) which generates voltage, an electrolytic cell consumes it. The power source acts as an "electron pump," pulling electrons away from the anode and pushing them to the cathode, thereby imposing the charge and driving the entire process.
Electrode Materials and Their Purpose
The material an electrode is made from is critical to the cell's function. The choice depends on whether the electrode should be an active participant or a passive observer in the reaction.
Inert Electrodes: The Passive Conductor
In many applications, the goal is to drive a reaction within the electrolyte without the electrode itself being consumed. In these cases, inert electrodes are used. Materials like platinum and graphite (a form of carbon) are common choices because they are excellent electrical conductors but are chemically resistant and unlikely to react.
Active Electrodes: The Reaction Participant
In some processes, such as electroplating or refining, the anode is intentionally designed to be consumed. An active electrode is made of a material that oxidizes and dissolves into the electrolyte, providing the metal ions that will later be deposited onto the cathode.
The Specialized Three-electrode System
For analytical chemistry, where the goal is to study a reaction rather than perform bulk electrolysis, a more sophisticated three-electrode system is required. This setup allows for extremely precise control over the electrode's potential.
The Working Electrode
This is the primary electrode where the reaction of interest occurs. Its electrical potential is the variable being carefully controlled and measured.
The Counter (or Auxiliary) Electrode
The counter electrode's sole purpose is to complete the electrical circuit. Current flows between the working electrode and the counter electrode, but the reactions happening at the counter electrode are not the focus of the experiment.
The Reference Electrode
This is the key to the system's precision. A reference electrode provides a stable, known electrical potential. No significant current flows through it, so its potential remains constant. By measuring the working electrode's potential against this stable reference, a researcher can know and control its voltage with high accuracy.
Understanding the Trade-offs
Choosing a system involves balancing simplicity against the need for control.
The Two-Electrode System: Simplicity for Production
This setup is simple, robust, and ideal for large-scale applications like producing chlorine gas or aluminum. Its limitation is a lack of precise potential control, as the applied voltage is divided unpredictably between the two electrodes and the electrolyte.
The Three-Electrode System: Precision for Analysis
This system offers exquisite control, which is essential for studying reaction mechanisms and performing sensitive electrochemical analysis. However, it is more complex and generally used for low-current laboratory work, not for industrial production.
Making the Right Choice for Your Goal
Your objective dictates the necessary electrode configuration.
- If your primary focus is bulk electrolysis or a simple demonstration (e.g., splitting water, electroplating): A two-electrode system (anode and cathode) made of appropriate materials is the correct and most efficient choice.
- If your primary focus is analytical measurement or studying a reaction mechanism: A three-electrode system (working, counter, reference) is essential for the precision and control required.
Ultimately, understanding the function of each electrode empowers you to select the right tools for the chemical task at hand.
Summary Table:
| Electrode System | Key Components | Primary Use Case |
|---|---|---|
| Two-Electrode | Anode (Oxidation), Cathode (Reduction) | Bulk Electrolysis, Electroplating, Industrial Production |
| Three-Electrode | Working, Counter, Reference Electrodes | Analytical Chemistry, Precise Potential Control, Reaction Studies |
Need expert advice on selecting the right electrodes for your electrochemical application? KINTEK specializes in high-quality lab equipment and consumables for all your laboratory needs. Whether you're setting up for industrial production or precise analytical work, our team can help you choose the optimal system to enhance your efficiency and accuracy. Contact us today to discuss your specific requirements!
Visual Guide
Related Products
- Copper Sulfate Reference Electrode for Laboratory Use
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Sheet Electrode for Battery Lab Applications
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Aluminum Foil Current Collector for Lithium Battery
People Also Ask
- Is there a difference in performance between wood plug and ceramic core copper sulfate electrodes? Speed vs. Durability Explained
- What is the expected lifespan of a copper sulfate reference electrode? Maximize Longevity with Proper Maintenance
- What are the advantages and disadvantages of the ceramic core type copper sulfate reference electrode?
- What are the advantages and disadvantages of the wood plug type copper sulfate reference electrode? Speed vs. Durability Explained
- How should a copper sulfate reference electrode be maintained? Ensure Accurate Electrochemical Measurements



















