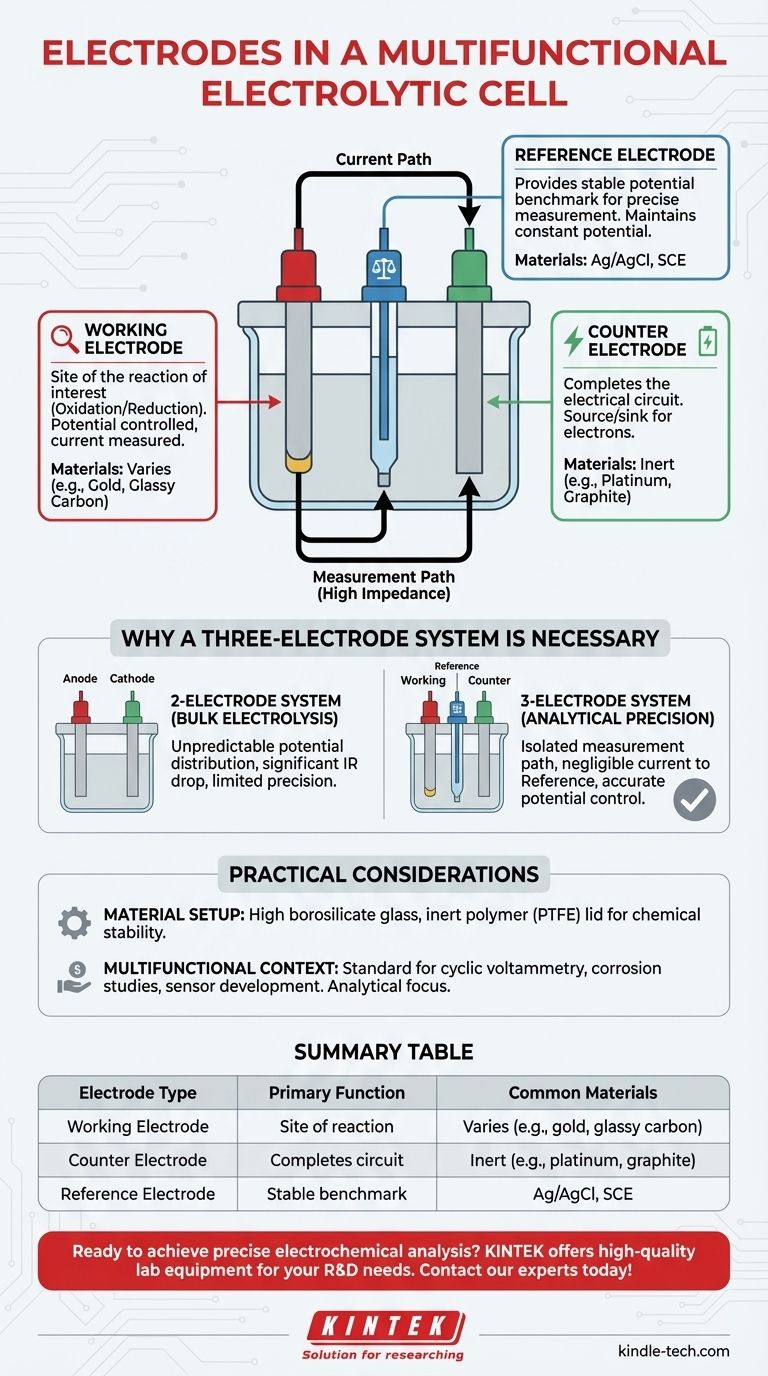
In short, a multifunctional electrolytic cell uses a three-electrode system. These electrodes are the working electrode, where the reaction of interest occurs; the counter electrode, which completes the electrical circuit; and the reference electrode, which provides a stable potential for precise measurements.
The use of a three-electrode system is the key feature that makes the cell "multifunctional." Unlike a simple two-electrode setup for bulk electrolysis, this configuration is designed for analytical precision, allowing researchers to accurately measure and control the potential at the working electrode, independent of the overall cell voltage.

The Role of Each Electrode in an Analytical System
A standard electrolytic cell uses two electrodes—an anode and a cathode—to drive a non-spontaneous reaction. A multifunctional cell adds a third to transform it into a precise measurement tool. Each has a distinct and critical job.
The Working Electrode (The Site of Interest)
The working electrode is the centerpiece of your experiment. It is the surface where the specific electrochemical reaction you want to study (be it oxidation or reduction) takes place.
Its potential is what you control, and its resulting current is what you measure. The material for this electrode is chosen based on the reaction being investigated.
The Counter Electrode (The Current Driver)
The counter electrode (also called the auxiliary electrode) acts as a source or sink for electrons to balance the reaction at the working electrode. Its sole purpose is to ensure current flows and the circuit is complete.
This electrode is typically made from an inert material, like graphite or platinum, that will not interfere with the primary reaction. It is the other half of the current-carrying pair, along with the working electrode.
The Reference Electrode (The Stable Benchmark)
The reference electrode is the most crucial component for accurate measurement. It maintains a constant, known electrochemical potential, acting as a stable benchmark.
It is placed close to the working electrode and is connected to a high-impedance voltmeter, meaning almost no current flows through it. This allows it to measure the exact potential of the working electrode without being affected by voltage drops elsewhere in the cell.
Why a Three-Electrode System is Necessary
The move from a simple two-electrode cell to a three-electrode system is driven by the need for accuracy and control in electrochemical analysis.
The Limitation of a Two-Electrode System
In a basic cell with only an anode and cathode, the voltage you apply from a power source is distributed unpredictably across several components: the anode, the cathode, and the electrolyte solution itself (known as IR drop).
You cannot know the precise potential at the surface of the electrode where your reaction of interest is happening. This makes it impossible to perform quantitative analysis or study reaction kinetics reliably.
The Precision of a Three-Electrode System
The three-electrode setup isolates the function of measurement from the function of driving current.
The current path is between the working and counter electrodes. The separate measurement path is between the working and reference electrodes. Because the reference electrode draws negligible current, its potential remains stable, giving you a true reading of the working electrode's potential.
Understanding the Practical Considerations
While powerful, this system's effectiveness depends on its proper implementation and understanding its context.
Material and Physical Setup
The cell's components are chosen for chemical inertness and stability. The body is often made of high borosilicate glass, with a lid of a non-reactive polymer like PTFE (Teflon).
This robust construction ensures that the cell itself does not contaminate or react with the electrolyte or the products of the electrochemical reaction, preserving the integrity of the experiment.
The "Multifunctional" Context
A three-electrode cell is not designed for industrial-scale production. Its purpose is analytical.
This setup is the standard for techniques like cyclic voltammetry, corrosion studies, and sensor development, where precise control and measurement of electrode potential are paramount to understanding the underlying electrochemical processes.
Making the Right Choice for Your Goal
The appropriate electrode configuration depends entirely on your objective.
- If your primary focus is bulk electrolysis (e.g., producing chlorine gas): A simpler two-electrode system (anode and cathode) is generally sufficient and more cost-effective.
- If your primary focus is electrochemical analysis (e.g., measuring reaction rates or concentrations): The three-electrode system (working, counter, reference) is essential for accurate and repeatable data.
- If your primary focus is material characterization (e.g., testing a new catalyst): The three-electrode configuration is the standard for quantifying the electrochemical properties of your material, which serves as the working electrode.
Ultimately, the three-electrode system empowers you to move beyond simply driving a reaction to precisely investigating and understanding it.
Summary Table:
| Electrode Type | Primary Function | Common Materials |
|---|---|---|
| Working Electrode | Site of the reaction being studied | Varies based on reaction (e.g., gold, glassy carbon) |
| Counter Electrode | Completes the electrical circuit | Inert materials (e.g., platinum, graphite) |
| Reference Electrode | Provides a stable potential benchmark | Silver/Silver Chloride (Ag/AgCl), Saturated Calomel (SCE) |
Ready to achieve precise electrochemical analysis in your lab? The right multifunctional electrolytic cell is key to accurate results. KINTEK specializes in high-quality lab equipment, including electrochemical cells and consumables, to support your research and development. Contact our experts today to find the perfect solution for your laboratory's needs and elevate your electrochemical experiments!
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Copper Sulfate Reference Electrode for Laboratory Use
- Gold Disc Electrode
People Also Ask
- What is a H type cell? A Guide to Divided Electrochemical Cells for Accurate Experiments
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What are the key features of a double-layer water-bath electrolytic cell? Achieve Precise Temperature Control for Your Experiments
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide


















